Melanocortin 4 receptor signaling in Sim1 neurons permits sexual receptivity in female mice
- PMID: 37033219
- PMCID: PMC10080118
- DOI: 10.3389/fendo.2023.983670
Melanocortin 4 receptor signaling in Sim1 neurons permits sexual receptivity in female mice
Abstract
Introduction: Female sexual dysfunction affects approximately 40% of women in the United States, yet few therapeutic options exist for these patients. The melanocortin system is a new treatment target for hypoactive sexual desire disorder (HSDD), but the neuronal pathways involved are unclear.
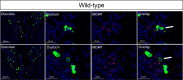
Methods: In this study, the sexual behavior of female MC4R knockout mice lacking melanocortin 4 receptors (MC4Rs) was examined. The mice were then bred to express MC4Rs exclusively on Sim1 neurons (tbMC4RSim1 mice) or on oxytocin neurons (tbMC4ROxt mice) to examine the effect on sexual responsiveness.
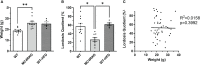
Results: MC4R knockout mice were found to approach males less and have reduced receptivity to copulation, as indicated by a low lordosis quotient. These changes were independent of body weight. Lordosis behavior was normalized in tbMC4RSim1 mice and improved in tbMC4ROxt mice. In contrast, approach behavior was unchanged in tbMC4RSim1 mice but greatly increased in tbMC4ROxt animals. The changes were independent of melanocortin-driven metabolic effects.
Discussion: These results implicate MC4R signaling in Oxt neurons in appetitive behaviors and MC4R signaling in Sim1 neurons in female sexual receptivity, while suggesting melanocortin-driven sexual function does not rely on metabolic neural circuits.
Keywords: MC4R; lordosis; melanocortin; obesity; oxytocin; sexual behavior; sim1; solicitation.
Copyright © 2023 Semple, Harberson, Xu, Rashleigh, Cartwright, Braun, Custer, Liu and Hill.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

