Food deprivation differentially modulates gene expression of LPXRFa and kisspeptin systems in the brain-pituitary axis of half-smooth tongue sole (Cynoglossus semilaevis)
- PMID: 37033260
- PMCID: PMC10081681
- DOI: 10.3389/fendo.2023.1099832
Food deprivation differentially modulates gene expression of LPXRFa and kisspeptin systems in the brain-pituitary axis of half-smooth tongue sole (Cynoglossus semilaevis)
Abstract
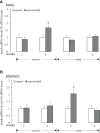
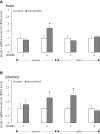
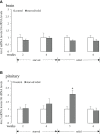
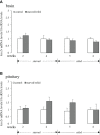
LPXRFa, also known as gonadotropin-inhibitory hormone (GnIH), and kisspeptin (Kiss) are two major hypothalamic peptides that modulate the reproductive axis of vertebrates, including teleosts. However, little information is available regarding the actions of nutritional status on the regulation of these two neuroendocrine systems in fish. Herein, we assessed the effects of starvation and refeeding on the expression of lpxrfa, kiss2 and their receptors (lpxrfa-r and kiss2r respectively) at the brain-pituitary level of half-smooth tongue sole (Cynoglossus semilaevis). Food deprivation for 4 weeks induced a rise in brain lpxrfa as well as brain and pituitary lpxrfa-r mRNA levels, and refeeding restored brain lpxrfa and lpxrfa-r expression back to normal. However, pituitary lpxrfa-r mRNA levels still remained high after 1 week of refeeding. Neither lpxrfa nor kiss2 transcripts in the pituitary were altered by fasting, but their mRNA levels increased significantly after 1 week of refeeding, and declined back to the control levels after 2 weeks of refeeding. None of brain kiss2 and kiss2r along with pituitary kiss2r transcripts were modified by the nutritional status. In summary, our results revealed an interaction between energy status and the elements of LPXRFa and Kiss systems in the brain-pituitary axis of half-smooth tongue sole. Food deprivation and refeeding differentially regulated the two systems, which provided additional evidence for the involvement of the LPXRFa and Kiss systems in the regulation of reproduction by energy balance in non-mammalian species.
Keywords: Cynoglossus semilaevis; LPXRFa; LPXRFa receptor; kisspeptin; kisspeptin receptor.
Copyright © 2023 Wang, Cui, Xu, Zhang, Jiang and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
In vitro effects of tongue sole LPXRFa and kisspeptin on relative abundance of pituitary hormone mRNA and inhibitory action of LPXRFa on kisspeptin activation in the PKC pathway.Anim Reprod Sci. 2019 Apr;203:1-9. doi: 10.1016/j.anireprosci.2019.01.009. Epub 2019 Feb 15. Anim Reprod Sci. 2019. PMID: 30797596
-
Inhibitory action of tongue sole LPXRFa, the piscine ortholog of gonadotropin-inhibitory hormone, on the signaling pathway induced by tongue sole kisspeptin in COS-7 cells transfected with their cognate receptors.Peptides. 2017 Sep;95:62-67. doi: 10.1016/j.peptides.2017.07.014. Epub 2017 Jul 25. Peptides. 2017. PMID: 28754347
-
Molecular characterization of Kiss2 receptor and in vitro effects of Kiss2 on reproduction-related gene expression in the hypothalamus of half-smooth tongue sole (Cynoglossus semilaevis).Gen Comp Endocrinol. 2017 Aug 1;249:55-63. doi: 10.1016/j.ygcen.2017.04.006. Epub 2017 Apr 21. Gen Comp Endocrinol. 2017. PMID: 28438528
-
The gonadotropin-inhibitory hormone (Lpxrfa) system's regulation of reproduction in the brain-pituitary axis of the zebrafish (Danio rerio).Biol Reprod. 2017 May 1;96(5):1031-1042. doi: 10.1093/biolre/iox032. Biol Reprod. 2017. PMID: 28430864 Review.
-
Recent studies of LPXRFa receptor signaling in fish and other vertebrates.Gen Comp Endocrinol. 2019 Jun 1;277:3-8. doi: 10.1016/j.ygcen.2018.11.011. Epub 2018 Nov 19. Gen Comp Endocrinol. 2019. PMID: 30465768 Review.
Cited by
-
Characterization of adaptive expression regulation of yellowtail kingfish (Seriola lalandi) leptin, receptor, and receptor overlapping transcript genes in response to fasting and re-feeding strategies.Fish Physiol Biochem. 2024 Aug;50(4):1513-1526. doi: 10.1007/s10695-024-01353-2. Epub 2024 May 9. Fish Physiol Biochem. 2024. PMID: 38722479
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

