Vitamin D improves hepatic steatosis in NAFLD via regulation of fatty acid uptake and β-oxidation
- PMID: 37033263
- PMCID: PMC10074590
- DOI: 10.3389/fendo.2023.1138078
Vitamin D improves hepatic steatosis in NAFLD via regulation of fatty acid uptake and β-oxidation
Abstract
Introduction: The study aimed to explore the association of serum 25(OH)D3 and hepatic steatosis in non-alcoholic fatty liver disease (NAFLD) patients and to determine whether the effect of vitamin D (VD) is mediated by activation of the peroxisome proliferator-activated receptor α (PPARα) pathway.
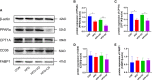
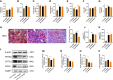
Methods: The study contained a case-control study, in vivo and in vitro experiments. A case-control study was conducted to compare serum parameters between NAFLD patients and controls and to evaluate the association of 25(OH)D3 and NAFLD. In vivo study, male Wistar rats were randomly divided into control and model groups, fed a standard chow diet and a high-fat diet (HFD), respectively, for 7 weeks to generate an NAFLD model. Then, the rats were treated with VD and a PPARα antagonist (MK886) for 7 weeks. Tissue and serum were collected and assessed by biochemical assays, morphological analysis, histological analysis, and western blot analysis. In vitro, HepG2 cells were incubated with oleic acid (OA) to induce steatosis, which was evaluated by staining. HepG2 cells were pretreated with MK886 followed by calcitriol treatment, and differences in lipid metabolism-related proteins were detected by western blot.
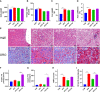
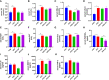
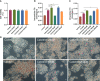
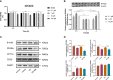
Results: NAFLD patients were characterized by impaired liver function, dyslipidemia, and insulin resistance. Serum 25(OH)D3 was negatively associated with alanine aminotransferase (ALT) in NAFLD. VD deficiency was a risk factor for patients with no advanced fibrosis. Adequate VD status (25(OH)D3 >20 ng/mL) had a protective effect in patients after adjustment for confounding variables. NAFLD rats showed hyperlipidemia with severe hepatic steatosis, systematic inflammation, and lower serum 25(OH)D3. VD treatment ameliorated hepatic steatosis both in NAFLD rats and OA-induced HepG2 cells. Further, MK886 inhibited the anti-steatosis effect of VD.
Conclusion: The study revealed that an adequate VD level may act as a protective factor in NAFLD and that VD may alleviate hepatic steatosis via the PPARα signaling pathway.
Keywords: PPARα; hepatic steatosis; lipid metabolism; non-alcoholic fatty liver disease; vitamin D.
Copyright © 2023 Du, Xiang, Zhang, Yang, Zhao, Li, Zhou and Ma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

