Preimplantation or gestation/lactation high-fat diet alters adult offspring metabolism and neurogenesis
- PMID: 37033334
- PMCID: PMC10077335
- DOI: 10.1093/braincomms/fcad093
Preimplantation or gestation/lactation high-fat diet alters adult offspring metabolism and neurogenesis
Abstract
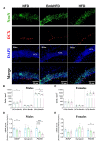
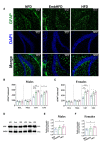
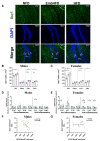
Poor maternal nutrition during pregnancy is known to impair fetal development. Moreover, the preimplantation period is vulnerable to adverse programming of disease. Here, we investigated the effect of a mouse maternal high-fat diet in healthy non-obese dams during preimplantation or throughout pregnancy and lactation on metabolism-related parameters and hippocampal neurogenesis in adult offspring. Female mice were fed from conception either a normal fat diet (normal fat diet group) or high-fat diet throughout gestation and lactation (high-fat diet group), or high-fat diet only during preimplantation (embryonic high-fat diet group, high-fat diet up to E3.5, normal fat diet thereafter). Maternal high-fat diet caused changes in the offspring, including increased systolic blood pressure, diurnal activity, respiratory quotient, and energy expenditure in high-fat diet females, and increased systolic blood pressure and respiratory quotient but decreased energy expenditure in high-fat diet males. High-fat diet males had a higher density of newborn neurons and a lower density of mature neurons in the dentate gyrus, indicating that exposure to a maternal high-fat diet may regulate adult neurogenesis. A maternal high-fat diet also increased the density of astrocytes and microglia in the hippocampus of high-fat diet males and females. Generally, a graded response (normal fat diet < embryonic high-fat < high-fat diet) was observed, with only 3 days of high-fat diet exposure altering offspring energy metabolism and hippocampal cell density. Thus, early maternal exposure to a fatty diet, well before neural differentiation begins and independently of maternal obesity, is sufficient to perturb offspring energy metabolism and brain physiology with lifetime consequences.
Keywords: brain development; metabolic health; nutrition; offspring long-term health; peri-conception.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures





References
-
- Collaborators GBDRF . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: A systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1923–1994. - PMC - PubMed
-
- Bianco-Miotto T, Craig JM, Gasser YP, van Dijk SJ, Ozanne SE. Epigenetics and DOHaD: From basics to birth and beyond. J Dev Orig Health Dis. 2017;8(5):513–519. - PubMed
-
- Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–417. - PubMed
-
- Gluckman PD, Hanson MA. Developmental origins of disease paradigm: A mechanistic and evolutionary perspective. Pediatr Res. 2004;56(3):311–317. - PubMed
LinkOut - more resources
Full Text Sources
