Impaired interactions of ataxin-3 with protein complexes reveals their specific structure and functions in SCA3 Ki150 model
- PMID: 37033372
- PMCID: PMC10080164
- DOI: 10.3389/fnmol.2023.1122308
Impaired interactions of ataxin-3 with protein complexes reveals their specific structure and functions in SCA3 Ki150 model
Abstract
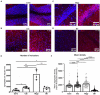
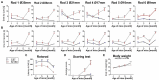
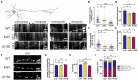
Spinocerebellar ataxia type 3 (SCA3/MJD) is a neurodegenerative disease caused by CAG expansion in mutant ATXN3 gene. The resulting PolyQ tract in mutant ataxin-3 protein is toxic to neurons and currently no effective treatment exists. Function of both normal and mutant ataxin-3 is pleiotropic by their interactions and the influence on protein level. Our new preclinical Ki150 model with over 150 CAG/Q in ataxin-3 has robust aggregates indicating the presence of a process that enhances the interaction between proteins. Interactions in large complexes may resemble the real-life inclusion interactions and was never examined before for mutant and normal ataxin-3 and in homozygous mouse model with long polyQ tract. We fractionated ataxin-3-positive large complexes and independently we pulled-down ataxin-3 from brain lysates, and both were followed by proteomics. Among others, mutant ataxin-3 abnormally interacted with subunits of large complexes such as Cct5 and 6, Tcp1, and Camk2a and Camk2b. Surprisingly, the complexes exhibit circular molecular structure which may be linked to the process of aggregates formation where annular aggregates are intermediate stage to fibrils which may indicate novel ataxin-3 mode of interactions. The protein complexes were involved in transport of mitochondria in axons which was confirmed by altered motility of mitochondria along SCA3 Ki150 neurites.
Keywords: CAG; SCA3; aggregates; ataxin-3; interactions; mouse mitochondria; neurodegeneration; polyQ.
Copyright © 2023 Piasecki, Wiatr, Ruszkowski, Marczak, Trottier and Figiel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Broad Influence of Mutant Ataxin-3 on the Proteome of the Adult Brain, Young Neurons, and Axons Reveals Central Molecular Processes and Biomarkers in SCA3/MJD Using Knock-In Mouse Model.Front Mol Neurosci. 2021 Jun 17;14:658339. doi: 10.3389/fnmol.2021.658339. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34220448 Free PMC article.
-
A new humanized ataxin-3 knock-in mouse model combines the genetic features, pathogenesis of neurons and glia and late disease onset of SCA3/MJD.Neurobiol Dis. 2015 Jan;73:174-88. doi: 10.1016/j.nbd.2014.09.020. Epub 2014 Oct 7. Neurobiol Dis. 2015. PMID: 25301414
-
Altered Levels of Proteins and Phosphoproteins, in the Absence of Early Causative Transcriptional Changes, Shape the Molecular Pathogenesis in the Brain of Young Presymptomatic Ki91 SCA3/MJD Mouse.Mol Neurobiol. 2019 Dec;56(12):8168-8202. doi: 10.1007/s12035-019-01643-4. Epub 2019 Jun 14. Mol Neurobiol. 2019. PMID: 31201651 Free PMC article.
-
Toward therapeutic targets for SCA3: Insight into the role of Machado-Joseph disease protein ataxin-3 in misfolded proteins clearance.Prog Neurobiol. 2015 Sep;132:34-58. doi: 10.1016/j.pneurobio.2015.06.004. Epub 2015 Jun 27. Prog Neurobiol. 2015. PMID: 26123252 Review.
-
Progress in pathogenesis studies of spinocerebellar ataxia type 1.Philos Trans R Soc Lond B Biol Sci. 1999 Jun 29;354(1386):1079-81. doi: 10.1098/rstb.1999.0462. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10434309 Free PMC article. Review.
Cited by
-
Behavioral Abnormalities, Cognitive Impairments, Synaptic Deficits, and Gene Replacement Therapy in a CRISPR Engineered Rat Model of 5p15.2 Deletion Associated With Cri du Chat Syndrome.Adv Sci (Weinh). 2025 Apr;12(14):e2415224. doi: 10.1002/advs.202415224. Epub 2025 Feb 18. Adv Sci (Weinh). 2025. PMID: 39965128 Free PMC article.
-
Allosteric Modulation of Pathological Ataxin-3 Aggregation: A Path to Spinocerebellar Ataxia Type-3 Therapies.bioRxiv [Preprint]. 2025 Jan 24:2025.01.22.633970. doi: 10.1101/2025.01.22.633970. bioRxiv. 2025. PMID: 39896516 Free PMC article. Preprint.
-
CAG-targeted brain-permeable therapy tested in biallelic humanized polyQ mouse models.Mol Ther Nucleic Acids. 2025 Feb 22;36(2):102496. doi: 10.1016/j.omtn.2025.102496. eCollection 2025 Jun 10. Mol Ther Nucleic Acids. 2025. PMID: 40171277 Free PMC article.
References
LinkOut - more resources
Full Text Sources

