Introduction of chirality at C1 position of 1-substituted-3,4-dihydroisoquinoline by its enantioselective reduction: synthesis of chiral 1-substituted-1,2,3,4-tetrahydroisoquinoline - a review
- PMID: 37033430
- PMCID: PMC10077949
- DOI: 10.1039/d3ra01413d
Introduction of chirality at C1 position of 1-substituted-3,4-dihydroisoquinoline by its enantioselective reduction: synthesis of chiral 1-substituted-1,2,3,4-tetrahydroisoquinoline - a review
Abstract
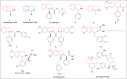
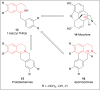
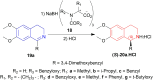
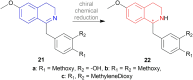
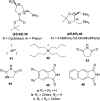
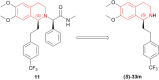
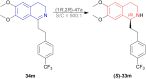
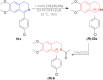
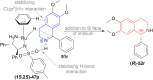
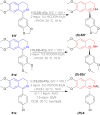
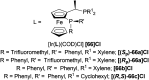
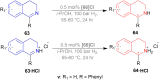
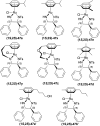
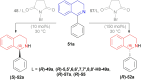
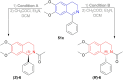
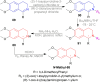
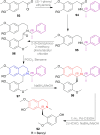
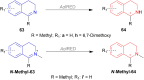
There is a wide range of biological activities associated with C1 chiral carbon containing 1-substituted-1,2,3,4-tetrahydroisoquinolines (1-substituted-THIQs) which constitute the isoquinoline alkaloids, a large group of natural products. This work summarizes several novel catalytic stereoselective approaches to enantioselectively reduce the 1-substituted-3,4-dihydroisoquinolines (1-substituted-DHIQs) to produce the desired 1-substituted-THIQs. The 1-substituted-DHIQs were prepared by using the Bischler-Napieralski reaction. The enantioselective reduction of 1-substituted-DHIQs was accomplished by using chiral hydride reducing agents, by hydrogenation with a chiral catalyst, by enantioselective reduction of DHIQs possessing a chiral auxiliary at the imine nitrogen by achiral metallic hydride reducing agents, or by enzymatic catalysis. Among these methods, much more work was carried out on the hydrogenation of 1-substituted-DHIQs in the presence of a chiral catalyst. This review summarizes articles and advancements on this topic from 1972 to 2023.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
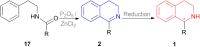




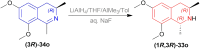



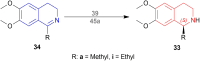




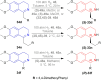
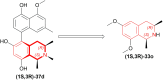


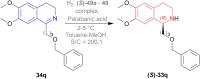
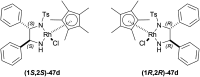





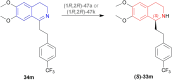

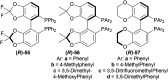

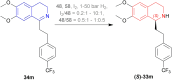
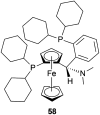

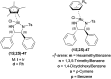


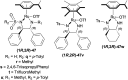





















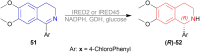





References
-
- Phillipson J. D., Roberts M. F. and Zenk M. H., The chemistry and biology of isoquinoline alkaloids, Springer Science & Business Media, 1985
-
- Scott J. D. Williams R. M. Chem. Rev. 2002;102:1669–1730. - PubMed
-
- Bentley K. W. Nat. Prod. Rep. 2006;23:444–463. - PubMed
-
- Shamma M., The isoquinoline alkaloids chemistry and pharmacology, Elsevier, 2012, vol. 25
-
- Manske R. H. F. and Holmes H. L., The alkaloids: chemistry and physiology, Elsevier, 2014
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

