Synthesis of magnetic graphene-like carbon nitride-cobalt ferrite (g-C3N4/CoFe2O4) nanocomposite for sonocatalytic remediation of toxic organic dyes
- PMID: 37033431
- PMCID: PMC10077340
- DOI: 10.1039/d3ra00057e
Synthesis of magnetic graphene-like carbon nitride-cobalt ferrite (g-C3N4/CoFe2O4) nanocomposite for sonocatalytic remediation of toxic organic dyes
Abstract
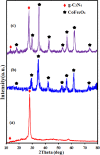
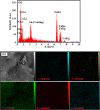
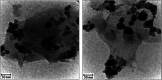
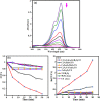
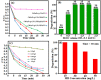
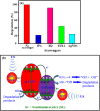
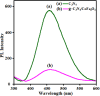
A novel magnetic g-C3N4/CoFe2O4 nanocomposite was successfully synthesized by a simple hydrothermal method and applied as a new graphene-like carbon nitride-based sonocatalyst for sonodegradation of pollutant dyes. The as-prepared samples were characterized by using X-ray diffraction (XRD), Fourier transform infrared (FT-IR) spectroscopy, field-emission scanning electron microscopy (FE-SEM), energy-dispersive X-ray spectroscopy (EDX), transmission electron microscopy (TEM), vibrating sample magnetometry (VSM), X-ray photoelectron spectroscopy (XPS), UV-visible diffuse reflectance spectroscopy (DRS), BET surface area measurements and photoluminescence (PL) spectroscopy. The results indicate that the nanocomposite sample is composed of spherical CoFe2O4 nanoparticles adhered to g-C3N4 naosheets. The g-C3N4/CoFe2O4 nanocomposites were used as a new magnetically separable sonocatalyst in H2O2-assisted sonodegradation of methylene blue (MB), rhodamine B (RhB) and methyl orange (MO) dyes in aqueous media. The results showed complete degradation (ca. 100%) of dyes within short times (30-35 min). The sonocatalytic activity of graphitic carbon nitride (g-C3N4) was greatly enhanced with CoFe2O4 modification. Trapping experiments indicated that the g-C3N4/CoFe2O4 nanocomposites serves as a generator of hydroxyl radical (˙OH) via activation of H2O2 for degradation of dyes under ultrasound irradiation. Furthermore, the magnetic sonocatalyst can be separated from solution by an external magnet and reused several times without observable loss of activity. The possible mechanism of sonocatalytic activity was also proposed according to experimental results.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures









References
-
- Saranya M. Ramachandran R. Samuel E. J. J. Jeong S. K. Grace A. N. Powder Technol. 2015;279:209–220.
-
- Ji K. Deng J. Zang H. Han J. Arandiyan H. Dai H. Appl. Catal., B. 2015;165:285–295.
-
- Bartolomeu M. Neves M. G. P. M. S. Faustino M. A. F. Almeida A. Photochem. Photobiol. Sci. 2018;17:1573–1598. - PubMed
-
- Kanakaraju D. Glass B. D. Oelgemöller M. J. Environ. Manage. 2018;219:189–207. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

