Maternal immunization with pneumococcal surface protein A provides the immune memories of offspring against pneumococcal infection
- PMID: 37033488
- PMCID: PMC10076723
- DOI: 10.3389/fcimb.2023.1059603
Maternal immunization with pneumococcal surface protein A provides the immune memories of offspring against pneumococcal infection
Abstract
Introduction: Streptococcus pneumoniae (S. pneumoniae) is one of the most widespread pathogens in the world and one of the largest infectious causes of infant mortality. Although current vaccines have various benefits, antibiotic resistance and the inability to vaccinate infants less than one year old demands the development of new protective strategies. One strategy, 'maternal immunization', is to protect infants by passive immunity from an immunized mother, although its mechanism is still not fully understood.
Materials and methods: The current study aimed to acquire immunity against S. pneumoniae in infants by maternal immunization with pneumococcal common antigen, pneumococcal surface protein A (PspA). Four-week-old female mice were immunized with recombinant PspA intranasally twice a week for three weeks. Females were mated with age-matched males after immunization, and delivered offspring.
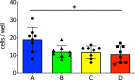
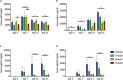
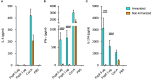
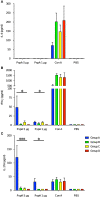
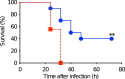
Results: The week-old offspring derived from and fostered by immunized mothers had more anti-PspA-specific antibody producing cells in the spleen than those derived from sham-immunized mothers. The offspring were raised up to four weeks old and were subcutaneously stimulated with recombinant PspA. The levels of anti-PspA IgG in sera after stimulation were significantly higher in the offspring derived from the immunized mothers and the induced specific antibody to PspA showed protective efficacy against systemic pneumococcal infection.
Discussion: Maternal immunization is suggested to be able to provide a sustained immune memory to offspring. The current study would be a milestone in the field of maternal immunization toward a universal pneumococcal vaccine.
Keywords: PspA; Streptococcus pneumoniae; immunological memory; invasive infection; maternal immunization.
Copyright © 2023 Kono, Iyo, Murakami, Sakatani, Nanushaj and Hotomi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Cabinian A., Sinsimer D., Tang M., Zumba O., Mehta H., Toma A., et al. (2016). Transfer of maternal immune cells by breastfeeding: Maternal cytotoxic T lymphocytes present in breast milk localize in the peyer's patches of the nursed infant. PloS One 11 (6), e0156762. doi: 10.1371/journal.pone.0156762 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

