Nirmatrelvir/ritonavir for patients with SARS-CoV-2 infection and impaired kidney function during the Omicron surge
- PMID: 37033654
- PMCID: PMC10073454
- DOI: 10.3389/fphar.2023.1147980
Nirmatrelvir/ritonavir for patients with SARS-CoV-2 infection and impaired kidney function during the Omicron surge
Abstract
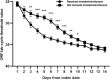
Background: Nirmatrelvir/ritonavir has demonstrated effectiveness in high-risk patients with coronavirus disease 2019 (COVID-19). However, investigations on the efficacy and safety of nirmatrelvir/ritonavir in patients with kidney dysfunction are limited. Methods: Data were collected from the patients admitted to a COVID-19 referral center in Shanghai, China. Patients were at least 18 years of age and had a baseline estimated glomerular filtration rate (eGFR) of <60 ml/min/1·73 m2. The primary endpoint was a composite of all-cause mortality, intensive care unit admission, or cardiovascular events. The secondary endpoint was viral shedding. Results: Among the 195 participants, 73 received nirmatrelvir/ritonavir. A lower risk of the primary endpoint was observed in nirmatrelvir/ritonavir recipients compared with non-recipients [adjusted HR 0.56 (95% CI: 0.32-0.96); p = 0.035]. Nirmatrelvir/ritonavir recipients experienced a shorter duration of viral shedding [adjusted HR 3·70 (95%CI: 2.60-5.28); p < 0.001) and faster viral load clearance versus non-recipients. Among the nirmatrelvir/ritonavir users, earlier initiation of nirmatrelvir/ritonavir within 5 days since COVID-19 diagnosis was related with shorter viral shedding time (adjusted HR 7.84 [95% CI: 3.28-18.76]; p < 0.001) compared to late initiation. No patients reported serious adverse events during treatment. Conclusion: Our findings support the early initiation of nirmatrelvir/ritonavir for high-risk patients with impaired kidney function. This could improve patient outcomes and shorten the viral shedding period.
Keywords: COVID-19; SARS-CoV-2; impaired kidney function; nirmatrelvir/ritonavir; omicron; outcomes.
Copyright © 2023 Yan, Cai, Wang, Zhu, Li, Li, Wu, Che, Gu and Mou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study.Lancet Infect Dis. 2022 Dec;22(12):1681-1693. doi: 10.1016/S1473-3099(22)00507-2. Epub 2022 Aug 24. Lancet Infect Dis. 2022. PMID: 36029795 Free PMC article.
-
Comparison of safety and efficacy between Nirmatrelvir-ritonavir and molnupiravir in the treatment of COVID-19 infection in patients with advanced kidney disease: a retrospective observational study.EClinicalMedicine. 2024 May 3;72:102620. doi: 10.1016/j.eclinm.2024.102620. eCollection 2024 Jun. EClinicalMedicine. 2024. PMID: 38737003 Free PMC article.
-
Analysis of All-Cause Hospitalization and Death Among Nonhospitalized Patients With Type 2 Diabetes and SARS-CoV-2 Infection Treated With Molnupiravir or Nirmatrelvir-Ritonavir During the Omicron Wave in Hong Kong.JAMA Netw Open. 2023 May 1;6(5):e2314393. doi: 10.1001/jamanetworkopen.2023.14393. JAMA Netw Open. 2023. PMID: 37204790 Free PMC article.
-
"Saving lives with nirmatrelvir/ritonavir one transplant patient at a time".Transpl Infect Dis. 2023 Apr;25(2):e14037. doi: 10.1111/tid.14037. Epub 2023 Feb 27. Transpl Infect Dis. 2023. PMID: 36847419 Free PMC article. Review.
-
Molnupiravir and Nirmatrelvir-Ritonavir: Oral Coronavirus Disease 2019 Antiviral Drugs.Clin Infect Dis. 2023 Jan 6;76(1):165-171. doi: 10.1093/cid/ciac180. Clin Infect Dis. 2023. PMID: 35245942 Free PMC article. Review.
Cited by
-
Ritonavir: 25 Years' Experience of Concomitant Medication Management. A Narrative Review.Infect Dis Ther. 2024 May;13(5):1005-1017. doi: 10.1007/s40121-024-00959-6. Epub 2024 Apr 12. Infect Dis Ther. 2024. PMID: 38609668 Free PMC article. Review.
-
Nirmatrelvir combined with ritonavir for preventing and treating COVID-19.Cochrane Database Syst Rev. 2023 Nov 30;11(11):CD015395. doi: 10.1002/14651858.CD015395.pub3. Cochrane Database Syst Rev. 2023. PMID: 38032024 Free PMC article.
-
Oral Agents and SARS-CoV-2 Vaccine Effectiveness against Severe COVID-19 Omicron Events in Patients Requiring Maintenance Dialysis.Kidney360. 2024 Mar 1;5(3):445-450. doi: 10.34067/KID.0000000000000373. Epub 2024 Feb 1. Kidney360. 2024. PMID: 38297444 Free PMC article. No abstract available.
-
Efficacy and safety of antiviral therapies for the treatment of persistent COVID-19 in immunocompromised patients since the Omicron surge: a systematic review.J Antimicrob Chemother. 2025 Mar 3;80(3):633-644. doi: 10.1093/jac/dkae482. J Antimicrob Chemother. 2025. PMID: 39804238 Free PMC article.
References
-
- Christensen P. A., Olsen R. J., Long S. W., Snehal R., Davis J. J., Ojeda Saavedra M., et al. (2022). Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with coronavirus disease 2019 caused by the omicron variant of severe acute respiratory syndrome coronavirus 2 in houston, Texas. Am. J. Pathol. 192 (4), 642–652. 10.1016/j.ajpath.2022.01.007 - DOI - PMC - PubMed
-
- Fact Sheet for Healthcare Providers (2022). Emergency use authorization for Paxlovid. Maryland: FDA.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

