Green Surfactants (Biosurfactants): A Petroleum-Free Substitute for Sustainability-Comparison, Applications, Market, and Future Prospects
- PMID: 37033812
- PMCID: PMC10077441
- DOI: 10.1021/acsomega.3c00591
Green Surfactants (Biosurfactants): A Petroleum-Free Substitute for Sustainability-Comparison, Applications, Market, and Future Prospects
Abstract
Surfactants are a group of amphiphilic molecules (i.e., having both hydrophobic and hydrophilic domains) that are a vital part of nearly every contemporary industrial process such as in agriculture, medicine, personal care, food, and petroleum. In general surfactants can be derived from (i) petroleum-based sources or (ii) microbial/plant origins. Petroleum-based surfactants are obvious results from petroleum products, which lead to petroleum pollution and thus pose severe problems to the environment leading to various ecological damages. Thus, newer techniques have been suggested for deriving surfactant molecules and maintaining environmental sustainability. Biosurfactants are surfactants of microbial or plant origins and offer much added advantages such as high biodegradability, lesser toxicity, ease of raw material availability, and easy applicability. Thus, they are also termed "green surfactants". In this regard, this review focused on the advantages of biosurfactants over the synthetic surfactants produced from petroleum-based products along with their potential applications in different industries. We also provided their market aspects and future directions that can be considered with selections of biosurfactants. This would open up new avenues for surfactant research by overcoming the existing bottlenecks in this field.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

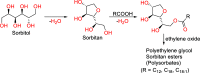

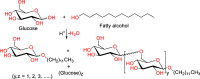

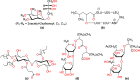



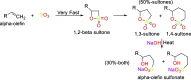










References
-
- Ricardo F.; Ruiz-Puentes P.; Reyes L. H.; Cruz J. C.; Alvarez O.; Pradilla D. Estimation and prediction of the air-water interfacial tension in conventional and peptide surface-active agents by random Forest regression. Chem. Eng. Sci. 2023, 265, 118208.10.1016/j.ces.2022.118208. - DOI
-
- De S.; Malik S.; Ghosh A.; Saha R.; Saha B. A review on natural surfactants. RSC Adv. 2015, 5 (81), 65757–65767. 10.1039/C5RA11101C. - DOI
-
- Kralova I.; Sjöblom J. Surfactants Used in Food Industry: A Review. J. Dispersion Sci. Technol. 2009, 30 (9), 1363–1383. 10.1080/01932690902735561. - DOI
-
- Kirtil E.; Oztop M. H. Mechanism of adsorption for design of role-specific polymeric surfactants. Chem. Pap. 2023, 10.1007/s11696-022-02636-9. - DOI
Publication types
LinkOut - more resources
Full Text Sources

