Cost-Effective Pipeline for a Rational Design and Selection of Capsaicin Analogues Targeting TRPV1 Channels
- PMID: 37033853
- PMCID: PMC10077575
- DOI: 10.1021/acsomega.2c05672
Cost-Effective Pipeline for a Rational Design and Selection of Capsaicin Analogues Targeting TRPV1 Channels
Abstract
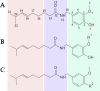
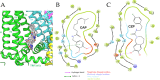
Transient receptor potential (TRP) channels constitute a large group of membrane receptors associated with sensory pathways in vertebrates. One of the most studied is TRPV1, a polymodal receptor tuned for detecting heat and pungent compounds. Specific inhibition of the nociceptive transduction at the peripheral nerve represents a convenient approach to pain relief. While acting as a chemoreceptor, TRPV1 shows high sensitivity and selectivity for capsaicin. In contrast to the drugs available on the market that target the inflammatory system, TRPV1 antagonists act as negative modulators of nociceptive transduction. Therefore, the development of compounds modulating TRPV1 activity has expanded dramatically over time. Experimental data suggest that most agonist and antagonist drugs interact at or near capsaicin's binding site. In particular, the properties of capsaicin's head play an essential role in modulating potency and affinity. Here, we explored a cost-efficient pipeline to predict the effects of introducing chemical modifications into capsaicin's head region. An extensive set of molecules was selected by first considering the geometrical properties of capsaicin's binding site and then molecular docking. Finally, the novel ligands were ranked by combining molecular and pharmacokinetic predictions.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Rosenbaum T.; Simon S. A.. TRPV1 Receptors and Signal Transduction; CRC Press/Taylor & Francis, 2007; ISBN 0849340489. - PubMed
LinkOut - more resources
Full Text Sources

