Tocilizumab-coated solid lipid nanoparticles loaded with cannabidiol as a novel drug delivery strategy for treating COVID-19: A review
- PMID: 37033914
- PMCID: PMC10073701
- DOI: 10.3389/fimmu.2023.1147991
Tocilizumab-coated solid lipid nanoparticles loaded with cannabidiol as a novel drug delivery strategy for treating COVID-19: A review
Abstract
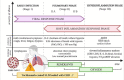
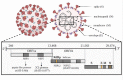
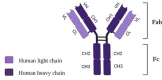
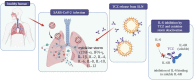
Commonly used clinical strategies against coronavirus disease 19 (COVID-19), including the potential role of monoclonal antibodies for site-specific targeted drug delivery, are discussed here. Solid lipid nanoparticles (SLN) tailored with tocilizumab (TCZ) and loading cannabidiol (CBD) are proposed for the treatment of COVID-19 by oral route. TCZ, as a humanized IgG1 monoclonal antibody and an interleukin-6 (IL-6) receptor agonist, can attenuate cytokine storm in patients infected with SARS-CoV-2. CBD (an anti-inflammatory cannabinoid and TCZ agonist) alleviates anxiety, schizophrenia, and depression. CBD, obtained from Cannabis sativa L., is known to modulate gene expression and inflammation and also shows anti-cancer and anti-inflammatory properties. It has also been recognized to modulate angiotensin-converting enzyme II (ACE2) expression in SARS-CoV-2 target tissues. It has already been proven that immunosuppressive drugs targeting the IL-6 receptor may ameliorate lethal inflammatory responses in COVID-19 patients. TCZ, as an immunosuppressive drug, is mainly used to treat rheumatoid arthritis, although several attempts have been made to use it in the active hyperinflammatory phase of COVID-19, with promising outcomes. TCZ is currently administered intravenously. It this review, we discuss the potential advances on the use of SLN for oral administration of TCZ-tailored CBD-loaded SLN, as an innovative platform for managing SARS-CoV-2 and related infections.
Keywords: COVID-19; cannabidiol (CBD); cytokine storm; oral drug therapy; solid lipid nanoparticles (SLN); tocilizumab (TCZ).
Copyright © 2023 Zielińska, Eder, Karczewski, Szalata, Hryhorowicz, Wielgus, Szalata, Dobrowolska, Atanasov, Słomski and Souto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
In search of preventive strategies: novel high-CBD Cannabis sativa extracts modulate ACE2 expression in COVID-19 gateway tissues.Aging (Albany NY). 2020 Nov 22;12(22):22425-22444. doi: 10.18632/aging.202225. Epub 2020 Nov 22. Aging (Albany NY). 2020. PMID: 33221759 Free PMC article.
-
Cannabidiol and SARS-CoV-2 Infection.Front Immunol. 2022 Mar 24;13:870787. doi: 10.3389/fimmu.2022.870787. eCollection 2022. Front Immunol. 2022. PMID: 35401543 Free PMC article.
-
Biomarker variation in patients successfully treated with tocilizumab for severe coronavirus disease 2019 (COVID-19): results of a multidisciplinary collaboration.Clin Exp Rheumatol. 2020 Jul-Aug;38(4):742-747. Epub 2020 Jun 23. Clin Exp Rheumatol. 2020. PMID: 32573419
-
Tocilizumab in SARS-CoV-2 Patients with the Syndrome of Cytokine Storm: A Narrative Review.Rev Recent Clin Trials. 2021;16(2):138-145. doi: 10.2174/1574887115666200917110954. Rev Recent Clin Trials. 2021. PMID: 32940187 Review.
-
Tocilizumab in patients with COVID-19: which patient, time, and dose?J Anesth. 2021 Dec;35(6):896-902. doi: 10.1007/s00540-021-02974-0. Epub 2021 Jul 15. J Anesth. 2021. PMID: 34264384 Free PMC article. Review.
Cited by
-
Anti-Inflammatory Effects of Cannabigerol In Vitro and In Vivo Are Mediated Through the JAK/STAT/NFκB Signaling Pathway.Cells. 2025 Jan 9;14(2):83. doi: 10.3390/cells14020083. Cells. 2025. PMID: 39851511 Free PMC article.
-
Cannabidiol as an immune modulator: A comprehensive review.Saudi Pharm J. 2025 May 23;33(3):11. doi: 10.1007/s44446-025-00005-7. Saudi Pharm J. 2025. PMID: 40407987 Free PMC article. Review.
-
Applications of nanoparticles in CAR-T cell therapy: non-viral manufacturing, enhancing in vivo function, and in vivo generation of CAR-T cells.Med Oncol. 2025 Jul 26;42(9):378. doi: 10.1007/s12032-025-02928-6. Med Oncol. 2025. PMID: 40715608 Review.
-
Perturbation of 3D nuclear architecture, epigenomic aging and dysregulation, and cannabinoid synaptopathy reconfigures conceptualization of cannabinoid pathophysiology: part 2-Metabolome, immunome, synaptome.Front Psychiatry. 2023 Oct 3;14:1182536. doi: 10.3389/fpsyt.2023.1182536. eCollection 2023. Front Psychiatry. 2023. PMID: 37854446 Free PMC article. Review.
-
Cannabidiol-Loaded Solid Lipid Nanoparticles Ameliorate the Inhibition of Proinflammatory Cytokines and Free Radicals in an In Vitro Inflammation-Induced Cell Model.Int J Mol Sci. 2024 Apr 26;25(9):4744. doi: 10.3390/ijms25094744. Int J Mol Sci. 2024. PMID: 38731964 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

