Non-viral TRAC-knocked-in CD19KICAR-T and gp350KICAR-T cells tested against Burkitt lymphomas with type 1 or 2 EBV infection: In vivo cellular dynamics and potency
- PMID: 37033919
- PMCID: PMC10081580
- DOI: 10.3389/fimmu.2023.1086433
Non-viral TRAC-knocked-in CD19KICAR-T and gp350KICAR-T cells tested against Burkitt lymphomas with type 1 or 2 EBV infection: In vivo cellular dynamics and potency
Abstract
Introduction: The ubiquitous Epstein-Barr virus (EBV) is an oncogenic herpes virus associated with several human malignancies. EBV is an immune-evasive pathogen that promotes CD8+ T cell exhaustion and dysregulates CD4+ T cell functions. Burkitt lymphoma (BL) is frequently associated with EBV infections. Since BL relapses after conventional therapies are difficult to treat, we evaluated prospective off-the-shelf edited CAR-T cell therapies targeting CD19 or the EBV gp350 cell surface antigen.
Methods: We used CRISPR/Cas9 gene editing methods to knock in (KI) the CD19CAR.CD28z or gp350CAR.CD28z into the T cell receptor (TCR) alpha chain (TRAC) locus.
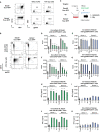
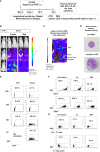
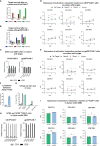
Results: Applying upscaled methods with the ExPERT ATx® MaxCyte system, KI efficacy was ~20% of the total ~2 × 108 TCR-knocked-out (KO) generated cells. KOTCRKICAR-T cells were co-cultured in vitro with the gp350+CD19+ BL cell lines Daudi (infected with type 1 EBV) or with Jiyoye (harboring a lytic type 2 EBV). Both types of CAR-T cells showed cytotoxic effects against the BL lines in vitro. CD8+ KICAR-T cells showed higher persistency than CD4+ KICAR-T cells after in vitro co-culture with BL and upregulation of the activation/exhaustion markers PD-1, LAG-3, and TIM-3. Two preclinical in vivo xenograft models were set up with Nod.Rag.Gamma mice injected intravenously (i.v.) with 2 × 105 Daudi/fLuc-GFP or with Jiyoye/fLuc-GFP cells. Compared with the non-treated controls, mice challenged with BL and treated with CD19KICAR-T cells showed delayed lymphoma dissemination with lower EBV DNA load. Notably, for the Jiyoye/fLuc-GFP model, almost exclusively CD4+ CD19KICAR-T cells were detectable at the endpoint analyses in the bone marrow, with increased frequencies of regulatory T cells (Tregs) and TIM-3+CD4+ T cells. Administration of gp350KICAR-T cells to mice after Jiyoye/GFP-fLuc challenge did not inhibit BL growth in vivo but reduced the EBV DNA load in the bone marrow and promoted gp350 antigen escape. CD8+PD-1+LAG-3+ gp350KICAR-T cells were predominant in the bone marrow.
Discussion: The two types of KOTCRKICAR-T cells showed different therapeutic effects and in vivo dynamics. These findings reflect the complexities of the immune escape mechanisms of EBV, which may interfere with the CAR-T cell property and potency and should be taken into account for future clinical translation.
Keywords: Burkitt lymphoma; CAR-T cell; CRISPR-Cas; EBV; gene editing; lymphoma; off-the-shelf; xenograft model.
Copyright © 2023 Braun, Pruene, Darguzyte, vom Stein, Nguyen, Wagner, Kath, Roig-Merino, Heuser, Riehm, Schneider, Awerkiew, Talbot, Bleich, Figueiredo, Bornhäuser and Stripecke.
Conflict of interest statement
Author AR-M is employed by MaxCyte Inc., MD, USA. Author RS has filed a patent application for generation of CAR-T cells targeting lytic herpes infections and is a founding shareholder and scientific consultant of BioSyngen/Zelltechs Lpt Ltd. Author DW has filed multiple patent applications on CRISPR-Cas gene editing and adoptive T cell therapy. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

