Pharmacological depletion of microglia alleviates neuronal and vascular damage in the diabetic CX3CR1-WT retina but not in CX3CR1-KO or hCX3CR1I249/M280-expressing retina
- PMID: 37033925
- PMCID: PMC10077890
- DOI: 10.3389/fimmu.2023.1130735
Pharmacological depletion of microglia alleviates neuronal and vascular damage in the diabetic CX3CR1-WT retina but not in CX3CR1-KO or hCX3CR1I249/M280-expressing retina
Abstract
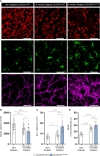
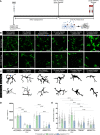
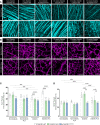
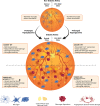
Diabetic retinopathy, a microvascular disease characterized by irreparable vascular damage, neurodegeneration and neuroinflammation, is a leading complication of diabetes mellitus. There is no cure for DR, and medical interventions marginally slow the progression of disease. Microglia-mediated inflammation in the diabetic retina is regulated via CX3CR1-FKN signaling, where FKN serves as a calming signal for microglial activation in several neuroinflammatory models. Polymorphic variants of CX3CR1, hCX3CR1I249/M280 , found in 25% of the human population, result in a receptor with lower binding affinity for FKN. Furthermore, disrupted CX3CR1-FKN signaling in CX3CR1-KO and FKN-KO mice leads to exacerbated microglial activation, robust neuronal cell loss and substantial vascular damage in the diabetic retina. Thus, studies to characterize the effects of hCX3CR1I249/M280 -expression in microglia-mediated inflammation in the diseased retina are relevant to identify mechanisms by which microglia contribute to disease progression. Our results show that hCX3CR1I249/M280 mice are significantly more susceptible to microgliosis and production of Cxcl10 and TNFα under acute inflammatory conditions. Inflammation is exacerbated under diabetic conditions and coincides with robust neuronal loss in comparison to CX3CR1-WT mice. Therefore, to further investigate the role of hCX3CR1I249/M280 -expression in microglial responses, we pharmacologically depleted microglia using PLX-5622, a CSF-1R antagonist. PLX-5622 treatment led to a robust (~70%) reduction in Iba1+ microglia in all non-diabetic and diabetic mice. CSF-1R antagonism in diabetic CX3CR1-WT prevented TUJ1+ axonal loss, angiogenesis and fibrinogen deposition. In contrast, PLX-5622 microglia depletion in CX3CR1-KO and hCX3CR1I249/M280 mice did not alleviate TUJ1+ axonal loss or angiogenesis. Interestingly, PLX-5622 treatment reduced fibrinogen deposition in CX3CR1-KO mice but not in hCX3CR1I249/M280 mice, suggesting that hCX3CR1I249/M280 expressing microglia influences vascular pathology differently compared to CX3CR1-KO microglia. Currently CX3CR1-KO mice are the most commonly used strain to investigate CX3CR1-FKN signaling effects on microglia-mediated inflammation and the results in this study indicate that hCX3CR1I249/M280 receptor variants may serve as a complementary model to study dysregulated CX3CR1-FKN signaling. In summary, the protective effects of microglia depletion is CX3CR1-dependent as microglia depletion in CX3CR1-KO and hCX3CR1I249/M280 mice did not alleviate retinal degeneration nor microglial morphological activation as observed in CX3CR1-WT mice.
Keywords: CX3CR1 chemokine receptor; depletion; diabetic retinopathy; inflammation; microglia.
Copyright © 2023 Church, Rodriguez, Mendiola, Vanegas, Gutierrez, Tamayo, Amadu, Velazquez, Cardona, Gyoneva, Cotleur, Ransohoff, Kaur and Cardona.
Conflict of interest statement
Author SG was employed by Biogen, Cambridge, MA, and is currently employed by Cerevel Therapeutics, Cambridge, MA. Author ACC is employed full-time by Biogen, Cambridge, MA. Author RR was employed full-time by Biogen, Cambridge, MA, and currently employed full-time by Third Rock Ventures, Boston, MA. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Ibrahim AS, El-Shishtawy MM, Peña A, Jr., Liou GI. Genistein attenuates retinal inflammation associated with diabetes by targeting of microglial activation. Mol Vis (2010) 16:2033–42. Available at: https://www.molvis.org/molvis/v16/a219/. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

