Arenaviruses: Old viruses present new solutions for cancer therapy
- PMID: 37033933
- PMCID: PMC10079900
- DOI: 10.3389/fimmu.2023.1110522
Arenaviruses: Old viruses present new solutions for cancer therapy
Abstract
Viral-based cancer therapies have tremendous potential, especially in the context of treating poorly infiltrated cold tumors. However, in tumors with intact anti-viral interferon (IFN) pathways, while some oncolytic viruses induce strong innate and adaptive immune responses, they are neutralized before exerting their therapeutic effect. Arenaviruses, particularly the lymphocytic choriomeningitis virus (LCMV) is a noncytopathic virus with preferential cancer tropism and evolutionary mechanisms to escape the immune system for longer and to block early clearance. These escape mechanisms include inhibition of the MAVS dependent IFN pathway and spike protein antigen masking. Regarding its potential for cancer treatment, LCMV is therefore able to elicit long-term responses within the tumor microenvironment (TME), boost anti-tumor immune responses and polarize poorly infiltrating tumors towards a hot phenotype. Other arenaviruses including the attenuated Junin virus vaccine also have anti-tumor effects. Furthermore, the LCMV and Pichinde arenaviruses are currently being used to create vector-based vaccines with attenuated but replicating virus. This review focuses on highlighting the potential of arenaviruses as anti-cancer therapies. This includes providing a molecular understanding of its tropism as well as highlighting past and present preclinical and clinical applications of noncytophatic arenavirus therapies and their potential in bridging the gap in the treatment of cancers weakly responsive or unresponsive to oncolytic viruses. In summary, arenaviruses represent promising new therapies to broaden the arsenal of anti-tumor therapies for generating an immunogenic tumor microenvironment.
Keywords: LCMV (lymphocytic choriomeningitis virus); arenaviruses; cold tumors; immunomodualtors; noncytopathic virus; virotherapy.
Copyright © 2023 Stachura, Stencel, Lu, Borkhardt and Pandyra.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

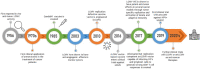
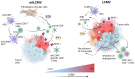
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

