The dysregulation of leukemia inhibitory factor and its implications for endometriosis pathophysiology
- PMID: 37033980
- PMCID: PMC10076726
- DOI: 10.3389/fimmu.2023.1089098
The dysregulation of leukemia inhibitory factor and its implications for endometriosis pathophysiology
Abstract
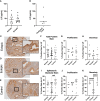
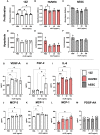
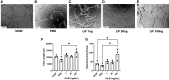
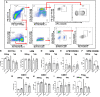
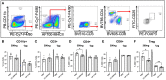
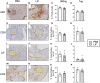
Endometriosis is an estrogen dominant, chronic inflammatory disease characterized by the growth of endometrial-like tissue outside of the uterus. The most common symptoms experienced by patients include manifestations of chronic pelvic pain- such as pain with urination, menstruation, or defecation, and infertility. Alterations to Leukemia Inhibitory Factor (LIF), a cytokine produced by the luminal and glandular epithelium of the endometrium that is imperative for successful pregnancy, have been postulated to contribute to infertility. Conditions such as recurrent implantation failure, unexplained infertility, and infertility associated diseases such as adenomyosis and endometriosis, have demonstrated reduced LIF production in the endometrium of infertile patients compared to fertile counterparts. While this highlights the potential involvement of LIF in infertility, LIF is a multifaceted cytokine which plays additional roles in the maintenance of cell stemness and immunomodulation. Thus, we sought to explore the implications of LIF production within ectopic lesions on endometriosis pathophysiology. Through immunohistochemistry of an endometrioma tissue microarray and ELISA of tissue protein extract and peritoneal fluid samples, we identify LIF protein expression in the ectopic lesion microenvironment. Targeted RT qPCR for LIF and associated signaling transcripts, identify LIF to be significantly downregulated in the ectopic tissue compared to eutopic and control while its receptor, LIFR, is upregulated, highlighting a discordance in ectopic protein and mRNA LIF expression. In vitro treatment of endometriosis representative cell lines (12Z and hESC) with LIF increased production of immune-recruiting cytokines (MCP-1, MCP-3) and the angiogenic factor, VEGF, as well as stimulated tube formation in human umbilical vein endothelial cells (HUVECs). Finally, LIF treatment in a syngeneic mouse model of endometriosis induced both local and peripheral alterations to immune cell phenotypes, ultimately reducing immunoregulatory CD206+ small peritoneal macrophages and T regulatory cells. These findings suggest that LIF is present in the ectopic lesions of endometriosis patients and could be contributing to lesion vascularization and immunomodulation.
Keywords: cytokine; endometriosis; immunomodulation; infertility; leukemia inhibitory factor (LIF); vascularization.
Copyright © 2023 Zutautas, Sisnett, Miller, Lingegowda, Childs, Bougie, Lessey and Tayade.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

