Anti-CD20 therapies in multiple sclerosis: From pathology to the clinic
- PMID: 37033984
- PMCID: PMC10076836
- DOI: 10.3389/fimmu.2023.1004795
Anti-CD20 therapies in multiple sclerosis: From pathology to the clinic
Abstract
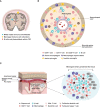
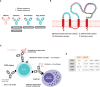
The immune system plays a significant role in multiple sclerosis. While MS was historically thought to be T cell-mediated, multiple pieces of evidence now support the view that B cells are essential players in multiple sclerosis pathogenic processes. High-efficacy disease-modifying therapies that target the immune system have emerged over the past two decades. Anti-CD20 monoclonal antibodies selectively deplete CD20+ B and CD20+ T cells and efficiently suppress inflammatory disease activity. These monotherapies prevent relapses, reduce new or active magnetic resonance imaging brain lesions, and lessen disability progression in patients with relapsing multiple sclerosis. Rituximab, ocrelizumab, and ofatumumab are currently used in clinical practice, while phase III clinical trials for ublituximab have been recently completed. In this review, we compare the four anti-CD20 antibodies in terms of their mechanisms of action, routes of administration, immunological targets, and pharmacokinetic properties. A deeper understanding of the individual properties of these molecules in relation to their efficacy and safety profiles is critical for their use in clinical practice.
Keywords: anti-CD20; multiple sclerosis; ocrelizumab; ofatumumab; rituximab; ublituximab.
Copyright © 2023 de Sèze, Maillart, Gueguen, Laplaud, Michel, Thouvenot, Zephir, Zimmer, Biotti and Liblau.
Conflict of interest statement
JS has received fees for serving on scientific advisory boards andconsulting, and research support from Novartis and Roche. EM has received research support from Fondation ARSEP and Biogen Idec, travel funding and/or consulting fees from Alexion, Biogen Idec, BMS, Merck, Novartis, Roche, Sanofi-Genzyme and Teva. AG has received personal compensation for consulting, lecturing and congress participation from Novartis, Alexon, AEI/Roche andNovartis. DL has received fees for board membership and consultancy and grants from Alexion, Actelion, BMS, Biogen,Merck, Novartis, Roche, Sanofi and Teva. LM has received consulting fees from Biogen, Novartis Pharma, Teva, BMS, Roche,Sanofi-Genzyme and Janssen Cilag. ET has received honoraria, travel grants, or research grants from the following pharmaceutical companies: Actelion, Biogen, Bristol Myers Squibb/Celgene, Merck, Novartis, Roche, Teva. HZ has received personal fees from Novartisrelated to this work and consulting fees from Biogen, Roche,Alexion, Roche, BMS, Novartis and Merck, unrelated to this work. DB has received personal compensation for consulting,serving on a scientific advisory board, speaking, or other activities with Biogen, Novartis, Merck, Sanofi-Genzyme, Roche, Celgene-BMS and Alexion. RL has received consultancy and speaker honoraria from Biogen, Sanofi-Genzyme, Merck-Serono,Novartis, Orion and GSK, and research grants from GSK, Population Bio and Roche. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

