Comparison of neoadjuvant immunotherapy versus routine neoadjuvant therapy for patients with locally advanced esophageal cancer: A systematic review and meta-analysis
- PMID: 37033991
- PMCID: PMC10076616
- DOI: 10.3389/fimmu.2023.1108213
Comparison of neoadjuvant immunotherapy versus routine neoadjuvant therapy for patients with locally advanced esophageal cancer: A systematic review and meta-analysis
Abstract
Background: The neoadjuvant use of immune checkpoint inhibitor combined with chemotherapy (nICT) or chemoradiotherapy (nICRT) in locally advanced esophageal cancer (EC) is currently an area of active ongoing research. Therefore, we carried out a comprehensive meta-analysis to compare the efficacy and safety of the new strategy with routine neoadjuvant strategy, which included neoadjuvant chemotherapy (nCT) and neoadjuvant chemoradiotherapy (nCRT).
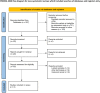
Patients and methods: MEDLINE (via PubMed), Embase (via OVID), ISI Web of Science database and Cochrane Library were included. And, all of them were searched for eligible studies between January, 2000 and February, 2023. The pathological complete response (pCR) and major pathological response (MPR) were primary outcome of our study. The second outcome of interest was R0 resection rate. Odds ratio (OR) and associated 95% CI were used as the effect indicators comparing the safety and efficiency of the neoadjuvant immunotherapy with the routine neoadjuvant therapy. Fixed-effect model (Inverse Variance) or random-effect model (Mantel-Haenszel method) was performed depending on the statistically heterogeneity.
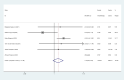
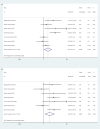
Results: There were eight trials with 652 patients were included in our meta-analysis. The estimated pCR rate was higher in the neoadjuvant immunotherapy group (OR =1.86; 95% CI, 1.25-2.75; I2 = 32.8%, P=0.166). The different results were found in the esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) subgroups, the estimated OR was 2.35 (95%CI, 1.00-2.72; I2 = 30.9%, P=0.215) in the EAC subgroup, and 2.35 (95% CI, 1.20-4.54; I2 = 45.3%, P=0.161) in the ESCC subgroup, respectively. The neoadjuvant immunotherapy also showed the advantage in the MPR rates (OR =2.66; 95% CI, 1.69-4.19; I2 = 24.3%, P=0.252). There was no obvious difference between the neoadjuvant immunotherapy and routine neoadjuvant therapy with respect to surgical resection rate, R0 resection rate, surgical delay rate; while more treatment-related adverse events were observed for the neoadjuvant immunotherapy for pneumonitis/pneumonia (OR=3.46, 95% CI, 1.31-9.16; I2 = 67.3%, P=0.005) and thyroid dysfunction (OR=4.69, 95% CI, 1.53-14.36; I2 = 56.5%, P=0.032).
Conclusion: The pooled correlations indicated that the neoadjuvant immunotherapy (both nICT and nICRT) could significantly increase the rates of pCR and MPR, compared with routine neoadjuvant therapy (both nCT and nCRT) in the treatment of locally advanced EC. The neoadjuvant immunotherapy and routine neoadjuvant therapy were with acceptable toxicity. However, randomized studies with larger groups of patients need to performed to confirm these results.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42020155802.
Keywords: chemoradiotherapy; chemotherapy; esophageal cancer; immune checkpoint inhibitor; meta-analysis; neoadjuvant; pathological complete response.
Copyright © 2023 Qin, Liu, Zhang, Liang, Mi, Yu, Xu, Li, Lin, Li, Tian and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Efficacy and safety of neoadjuvant immunotherapy combined with chemoradiotherapy or chemotherapy in esophageal cancer: A systematic review and meta-analysis.Front Immunol. 2023 Jan 24;14:1117448. doi: 10.3389/fimmu.2023.1117448. eCollection 2023. Front Immunol. 2023. PMID: 36761760 Free PMC article.
-
Neoadjuvant immune checkpoint inhibitor in combination with chemotherapy or chemoradiotherapy in resectable esophageal cancer: A systematic review and meta-analysis.Front Immunol. 2022 Sep 13;13:998620. doi: 10.3389/fimmu.2022.998620. eCollection 2022. Front Immunol. 2022. PMID: 36177019 Free PMC article.
-
The efficacy and safety of neoadjuvant immunotherapy in resectable locally advanced esophageal squamous cell carcinoma: A systematic review and meta-analysis.Front Immunol. 2023 Feb 17;14:1118902. doi: 10.3389/fimmu.2023.1118902. eCollection 2023. Front Immunol. 2023. PMID: 36875107 Free PMC article.
-
Comparison of efficacy and safety between neoadjuvant chemotherapy and neoadjuvant immune checkpoint inhibitors combined with chemotherapy for locally advanced esophageal squamous cell carcinoma: a systematic review and meta-analysis.Int J Surg. 2024 Jan 1;110(1):490-506. doi: 10.1097/JS9.0000000000000816. Int J Surg. 2024. PMID: 37800587 Free PMC article.
-
Pathologic responses and surgical outcomes after neoadjuvant immunochemotherapy versus neoadjuvant chemoradiotherapy in patients with locally advanced esophageal squamous cell carcinoma.Front Immunol. 2022 Nov 17;13:1052542. doi: 10.3389/fimmu.2022.1052542. eCollection 2022. Front Immunol. 2022. PMID: 36466925 Free PMC article.
Cited by
-
Neoadjuvant arterial infusion chemotherapy combined with immunotherapy in treating locally advanced lower esophageal and esophagogastric junction cancer.J Thorac Dis. 2025 Apr 30;17(4):2273-2285. doi: 10.21037/jtd-24-1908. Epub 2025 Apr 23. J Thorac Dis. 2025. PMID: 40400978 Free PMC article.
-
The efficacy of neoadjuvant immunotherapy in gastric cancer, adenocarcinoma of the esophagogastric junction, and esophageal cancer: a meta-analysis.Front Oncol. 2024 Nov 22;14:1502611. doi: 10.3389/fonc.2024.1502611. eCollection 2024. Front Oncol. 2024. PMID: 39650059 Free PMC article.
-
ISCU-p53 axis orchestrates macrophage polarization to dictate immunotherapy response in esophageal squamous cell carcinoma.Cell Death Dis. 2025 Jun 20;16(1):462. doi: 10.1038/s41419-025-07787-7. Cell Death Dis. 2025. PMID: 40541964 Free PMC article.
-
Feasibility and Safety of PD-1 Blockades Among Elderly Patients with Metastatic Esophageal Squamous Cell Carcinoma: A Real-World Study.Drug Des Devel Ther. 2024 Sep 16;18:4135-4151. doi: 10.2147/DDDT.S476457. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39308693 Free PMC article.
-
Prospects of immune checkpoint inhibitor in combination with chemotherapy or chemoradiation in esophago-gastric cancers.J Thorac Dis. 2025 Apr 30;17(4):1790-1794. doi: 10.21037/jtd-2024-2157. Epub 2025 Apr 27. J Thorac Dis. 2025. PMID: 40400948 Free PMC article. No abstract available.
References
-
- Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. . Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (Neocrtec5010): A phase iii multicenter, randomized, open-label clinical trial. J Clin Oncol Off J Am Soc Clin Oncol (2018) 36(27):2796–803. doi: 10.1200/jco.2018.79.1483 - DOI - PMC - PubMed
-
- Eyck BM, van Lanschot JJB, Hulshof M, van der Wilk BJ, Shapiro J, van Hagen P, et al. . Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: The randomized controlled cross trial. J Clin Oncol Off J Am Soc Clin Oncol (2021) 39(18):1995–2004. doi: 10.1200/jco.20.03614 - DOI - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

