Outcomes of Patients With Classic Hodgkin Lymphoma Who Relapsed After Autologous Stem Cell Transplant
- PMID: 37034004
- PMCID: PMC10079336
- DOI: 10.1097/HS9.0000000000000869
Outcomes of Patients With Classic Hodgkin Lymphoma Who Relapsed After Autologous Stem Cell Transplant
Abstract
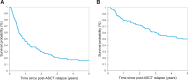
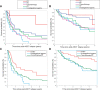
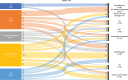
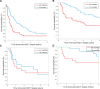
Immune checkpoint inhibitors (ICIs) and brentuximab vedotin (BV) are novel agents for classic Hodgkin lymphoma, including relapse after autologous stem cell transplant (ASCT). However, their impact on survival post-ASCT relapse, in comparison with conventional therapy, is less known due to the lack of randomized controlled trials. Clinical characteristics and outcomes of 115 patients with relapse (or progression) after ASCT are studied. After a median follow-up of 8.59 years from post-ASCT relapse, the median progression-free survival (PFS) and overall survival (OS) were 0.91 and 5.07 years, respectively. Median lines of therapy after post-ASCT relapse was 2 (range, 1-12). The median PFS was not reached (NR) versus 1.11 versus 0.50 versus 0.85 versus 0.78 years (P = 0.006) and OS was NR versus 7.60 versus 3.08 versus 3.51 versus 3.17 years (P = 0.28) in patients first treated with ICIs versus BV versus investigational agents versus chemotherapy versus radiation therapy (RT). First-line treatment with novel agents (ie, ICIs and BV) was associated with superior outcomes compared with investigational agents and chemotherapy/RT with a median PFS of 1.65 versus 0.50 versus 0.79 years (P = 0.003) and a median OS of 7.60 versus 3.08 versus 3.32 years (P = 0.08). Regardless of lines of therapy, the treatment with ICIs had the most favorable outcome with a median PFS and OS of 3.98 and NR years, respectively. Allogeneic stem cell transplant (allo-SCT) was done in 23 patients (20%), and the median post-allo-SCT PFS and OS were 1.31 and 2.35 years, respectively. In conclusion, survival following post-ASCT relapse improves significantly when patients receive novel agents.
Copyright © 2023 the Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the European Hematology Association.
Conflict of interest statement
TH reports Data Monitoring Committee: Seagen, Tess Therapeutics, Eli Lilly & Co. Scientific Advisory Board: Morphosys, Incyte, Biegene, Loxo Oncology. Research support: Genentech, Sorrento, BMS. TH receives no compensation for these activities, institution receives compensation. Research funding (to institution): Incyte, InnoCare, LOXO Oncology, Eli Lilly, MorphoSys, Novartis, Genentech, Genmab. Advisory board (compensation to institution): Eli Lilly, LOXO Oncology, TG Therapeutics, Incyte, InnoCare, Kite, Jansen, BeiGene. Honorarium (to institution): Kite. SMA is an Associate Editor of HemaSphere. All the other authors have no conflicts of interest to disclose.
Figures


References
-
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. - PubMed
-
- Ansell SM. Hodgkin lymphoma: a 2020 update on diagnosis, risk-stratification, and management. Am J Hematol. 2020;95:978–989. - PubMed
-
- André MPE, Girinsky T, Federico M, et al. Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2017;35:1786–1794. - PubMed
-
- Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372:1598–1607. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
