Intravesical aminoglycoside instillations as prophylaxis for recurrent urinary tract infection: patient satisfaction, long-term safety and efficacy
- PMID: 37034119
- PMCID: PMC10077025
- DOI: 10.1093/jacamr/dlad040
Intravesical aminoglycoside instillations as prophylaxis for recurrent urinary tract infection: patient satisfaction, long-term safety and efficacy
Abstract
Background: Recurrent urinary tract infections (UTIs) are common, especially in women. When oral antimicrobial prophylaxis is ineffective or not possible due to allergies or antimicrobial resistance, intravesical aminoglycoside instillations (IAIs) are a non-systemic alternative.
Objectives: To assess treatment satisfaction, long-term safety and efficacy of IAIs for recurrent UTI.
Methods: We conducted a cohort study using data collected between January 2013 and June 2022 at the Leiden University Medical Center. Adult patients with recurrent UTI who received prophylactic IAI were eligible for inclusion. Treatment satisfaction was assessed through a survey. Data on serum aminoglycoside concentrations, cystoscopy results and number of recurrences were obtained through chart review. Number of recurrences and UTI characteristics were compared between patients on and off IAI using Poisson and logistic mixed effects models.
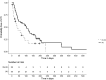
Results: Forty-four patients were included (median follow-up time 976 days) and 323 UTIs occurred during follow-up. Overall treatment satisfaction was high (median 79.2/100). All but one patient had undetectable serum aminoglycoside levels and no malignancies were found on follow-up cystoscopy. IAI increased the time to first recurrence (102 days versus 36 days, P = 0.02), reduced the number of recurrences (rate ratio 0.75, 95% CI 0.56-0.99, P = 0.04) and the necessity for systemic antibiotics (OR 0.33, 95% CI 0.13-0.86, P = 0.02).
Conclusions: In patients with recurrent UTI, IAI was associated with high treatment satisfaction, and was found to be a safe and effective alternative to oral antimicrobial prophylaxis.
© The Author(s) 2023. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures

Similar articles
-
Neomycin-polymyxin or gentamicin bladder instillations decrease symptomatic urinary tract infections in neurogenic bladder patients on clean intermittent catheterization.J Pediatr Urol. 2019 Apr;15(2):178.e1-178.e7. doi: 10.1016/j.jpurol.2018.12.001. Epub 2018 Dec 12. J Pediatr Urol. 2019. PMID: 30611650
-
Intravesical gentamicin instillation for the treatment and prevention of urinary tract infections in complex paediatric urology patients: evidence for safety and efficacy.J Pediatr Urol. 2021 Feb;17(1):65.e1-65.e11. doi: 10.1016/j.jpurol.2020.08.007. Epub 2020 Aug 19. J Pediatr Urol. 2021. PMID: 33309610
-
Intravesical Antibiotic Administration in the Treatment of Recurrent Urinary Tract Infections: Promising Results From a Case Series.Female Pelvic Med Reconstr Surg. 2020 Feb;26(2):152-154. doi: 10.1097/SPV.0000000000000810. Female Pelvic Med Reconstr Surg. 2020. PMID: 31990805
-
Are Intravesical Aminoglycosides the New Gold Standard in the Management of Refractory Urinary Tract Infection: A Systematic Review of Literature.J Clin Med. 2022 Sep 27;11(19):5703. doi: 10.3390/jcm11195703. J Clin Med. 2022. PMID: 36233570 Free PMC article. Review.
-
Intravesical Therapies for Recurrent Urinary Tract Infections: A Systematic Review.Cureus. 2024 Oct 23;16(10):e72175. doi: 10.7759/cureus.72175. eCollection 2024 Oct. Cureus. 2024. PMID: 39507191 Free PMC article. Review.
Cited by
-
Elimination and penetration of amikacin into urine in patients with decreased glomerular filtration rate.Clin Kidney J. 2024 Jan 3;17(1):sfae002. doi: 10.1093/ckj/sfae002. eCollection 2024 Jan. Clin Kidney J. 2024. PMID: 38260825 Free PMC article.
-
Gentamicin loaded niosomes against intracellular uropathogenic Escherichia coli strains.Sci Rep. 2024 May 3;14(1):10196. doi: 10.1038/s41598-024-59144-x. Sci Rep. 2024. PMID: 38702355 Free PMC article.
-
SWOT and Root Cause Analyses of Antimicrobial Resistance to Oral Antimicrobial Treatment of Cystitis.Antibiotics (Basel). 2024 Apr 4;13(4):328. doi: 10.3390/antibiotics13040328. Antibiotics (Basel). 2024. PMID: 38667004 Free PMC article. Review.
-
The Effects of Gentamicin Intravesical Bladder Instillations on Decreasing Urinary Tract Infections After Spinal Cord Injury and Disease.Neurourol Urodyn. 2025 Apr;44(4):829-838. doi: 10.1002/nau.70037. Epub 2025 Mar 17. Neurourol Urodyn. 2025. PMID: 40095724 Free PMC article.
References
-
- European Association of Urology . EAU guidelines on urological infections. 2021. https://uroweb.org/guidelines/urological-infections
LinkOut - more resources
Full Text Sources
