Correlation of serum SARS-CoV-2 IgM and IgG serology and clinical outcomes in COVID-19 patients: Experience from a tertiary care centre
- PMID: 37034133
- PMCID: PMC10080546
- DOI: 10.4331/wjbc.v14.i2.52
Correlation of serum SARS-CoV-2 IgM and IgG serology and clinical outcomes in COVID-19 patients: Experience from a tertiary care centre
Abstract
Background: The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus has become a pandemic for the last 2 years. Inflammatory response to the virus leads to organ dysfunction and death. Predicting the severity of inflammatory response helps in managing critical patients using serology tests IgG and IgM.
Aim: To investigate the correlation of the serology (IgM and IgG) with reverse transcriptase polymerase chain reaction (RT-PCR) status, disease severity [mild to critical], intensive care unit (ICU) admission, septic shock, acute kidney injury, and in-hospital mortality.
Methods: We conducted a longitudinal study to correlate serum SARS-CoV-2 immunoglobulin M (IgM) and immunoglobulin G (IgG) serology with clinical outcomes in coronavirus disease 2019 (COVID-19) patients. We analyzed patient data from March to December 2020 for those who were admitted at All India Institute of Medical Sciences Rishikesh. Clinical and laboratory data of these patients were collected from the e-hospital portal and analyzed. A correlation was seen with clinical outcomes and was assessed using MS Excel 2010 and SPSS software.
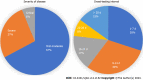
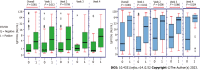
Results: Out of 494 patients, the mean age of patients was 48.95 ± 16.40 years and there were more male patients in the study (66.0%). The patients were classified as mild-moderate 328 (67.1%), severe 131 (26.8%), and critical 30 (6.1%). The mean duration from symptom onset to serology testing was 19.87 ± 30.53 d. In-hospital mortality was observed in 25.1% of patients. The seropositivity rate (i.e., either IgG or IgM > 10 AU) was 50%. IgM levels (AU/mL) (W = 33428.000, P ≤ 0.001) and IgG levels (AU/mL) (W = 39256.500, P ≤ 0.001), with the median IgM/ IgG levels (AU/mL), were highest in the RT-PCR-Positive group compared to RT-PCR-Negative clinical COVID-19. There was no significant difference between the two groups in terms of all other clinical outcomes (disease severity, septic shock, ICU admission, mechanical ventilation, and mortality).
Conclusion: The study showed that serology levels are high in RT-PCR positive group compared to clinical COVID-19. However, serology cannot be useful for the prediction of disease outcomes. The study also highlights the importance of doing serology at a particular time as antibody titers vary with the duration of the disease. In week intervals there was a significant correlation between clinical outcomes and serology on week 3.
Keywords: Inflammatory response; Reverse transcription polymerase chain reaction; SARS-CoV-2; Serology IgM and IgG.
©The Author(s) 2023. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: We declare that we have no conflicts of interest and it’s not funded.
Figures

Similar articles
-
Longitudinal Monitoring of SARS-CoV-2 IgM and IgG Seropositivity to Detect COVID-19.J Appl Lab Med. 2020 Sep 1;5(5):908-920. doi: 10.1093/jalm/jfaa079. J Appl Lab Med. 2020. PMID: 32428207 Free PMC article.
-
Prevalence of Sars-Cov-2 Infection in Health Workers (HWs) and Diagnostic Test Performance: The Experience of a Teaching Hospital in Central Italy.Int J Environ Res Public Health. 2020 Jun 19;17(12):4417. doi: 10.3390/ijerph17124417. Int J Environ Res Public Health. 2020. PMID: 32575505 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
[SARS-CoV-2 and Microbiological Diagnostic Dynamics in COVID-19 Pandemic].Mikrobiyol Bul. 2020 Jul;54(3):497-509. doi: 10.5578/mb.69839. Mikrobiyol Bul. 2020. PMID: 32755524 Review. Turkish.
-
Diagnostic Approaches for COVID-19 and Its Associated Complications.Diagnostics (Basel). 2021 Nov 9;11(11):2071. doi: 10.3390/diagnostics11112071. Diagnostics (Basel). 2021. PMID: 34829418 Free PMC article. Review.
Cited by
-
A novel highly specific biotinylated MAC-ELISA for detection of anti-SARS-CoV-2 nucleocapsid antigen IgM antibodies during the acute phase of COVID-19.Braz J Microbiol. 2023 Dec;54(4):2893-2901. doi: 10.1007/s42770-023-01160-6. Epub 2023 Nov 6. Braz J Microbiol. 2023. PMID: 37930615 Free PMC article.
References
-
- COVID-19 Treatment Guidelines Panel. National Institutes of Health; 2022. Coronavirus Disease 2019 [COVID-19] Treatment Guidelines; August 8, 2022 [cited August 16, 2022]. Available from: https://www.COVID-1919treatmentguidelines.nih.gov/ - PubMed
-
- COVID-19 [Internet]. CDC; 2020. Assessing Risk Factors; November 30, 2020 [Cited August 16, 2022]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/COVID-19-data/investigations-d... as-sessing-risk-factors.html.
-
- Udwadia ZF, Tripathi AR, Nanda VJ, Joshi SR. Prognostic Factors for Adverse Outcomes in COVID-19 Infection. J Assoc Physicians India. 2020;68:62–66. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

