Activation of POMC neurons to adiponectin participating in EA-mediated improvement of high-fat diet IR mice
- PMID: 37034166
- PMCID: PMC10077892
- DOI: 10.3389/fnins.2023.1145079
Activation of POMC neurons to adiponectin participating in EA-mediated improvement of high-fat diet IR mice
Abstract
Background: Insulin resistance (IR) is one of the common pathological manifestations of metabolic-related diseases, and the prevalence of relevant diseases is high. Acupuncture is beneficial to IR patients, but the central mechanism underlying this treatment remains unclear. This study provides mechanistic insights into how electroacupuncture (EA) improves IR through the response of Pro-opiomelanocortin (POMC) neurons to adiponectin (Adipo).
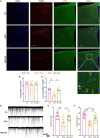
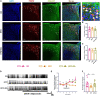
Methods: Glucose tolerance tests (GTT), Insulin tolerance tests (ITT) and fasting blood glucose (FBG) were detected by glucometer. Serum insulin, Adipo and skeletal muscle adiponectin receptor 1 (AdipoR1) protein levels were examined by ELISA. Homeostasis model assessment estimated insulin resistance (HOMA-IR) was calculated using the following formula: HOMA-IR = fasting insulin (FINS) (mU/L) × FBG (mmol/L)/22.5. The expression levels of AdipoR1 and Adipo mRNA in skeletal muscle were detected by real-time PCR quantification. The co-marking of c-Fos/AdipoR1 and POMC neurons were investigated using immunofluorescence. Spontaneous excitatory postsynaptic currents (sEPSCs) of POMC neurons and the response of POMC neurons to Adipo were detected via electrophysiology.
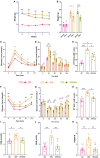
Results: EA significantly ameliorated HFD-induced impairment of GTT, ITT, FBG, and HOMA-IR which was correlated with recovery of the expression level of AdipoR1 and Adipo in skeletal muscle. The improved response of POMC neurons to Adipo in the hypothalamus may be a key factor in correcting abnormal glucose tolerance and improving IR.
Conclusion: This study demonstrates that EA can ameliorate HFD-induced impaired glucose tolerance through improved response of POMC neurons to Adipo in the hypothalamus, providing insight into the central mechanism of improving IR through EA.
Keywords: POMC; ZuSanLi; adiponectin; electroacupuncture; insulin resistance.
Copyright © 2023 Xu, Li, Ji, Fang, Yao, Xu and Yi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Benrick A., Maliqueo M., Johansson J., Sun M., Wu X., Mannerås-Holm L., et al. (2014). Enhanced insulin sensitivity and acute regulation of metabolic genes and signaling pathways after a single electrical or manual acupuncture session in female insulin-resistant rats. Acta Diabetol. 51 963–972. 10.1007/s00592-014-0645-4 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

