Crizotinib induces Par-4 secretion from normal cells and GRP78 expression on the cancer cell surface for selective tumor growth inhibition
- PMID: 37034206
- PMCID: PMC10077052
Crizotinib induces Par-4 secretion from normal cells and GRP78 expression on the cancer cell surface for selective tumor growth inhibition
Abstract
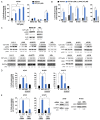
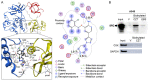
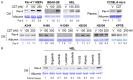
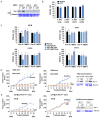
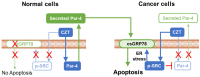
Lung cancer is the leading cause of cancer-related deaths. Lung cancer cells develop resistance to apoptosis by suppressing the secretion of the tumor suppressor Par-4 protein (also known as PAWR) and/or down-modulating the Par-4 receptor GRP78 on the cell surface (csGRP78). We sought to identify FDA-approved drugs that elevate csGRP78 on the surface of lung cancer cells and induce Par-4 secretion from the cancer cells and/or normal cells in order to inhibit cancer growth in an autocrine or paracrine manner. In an unbiased screen, we identified crizotinib (CZT), an inhibitor of activated ALK/MET/ROS1 receptor tyrosine kinase, as an inducer of csGRP78 expression in ALK-negative, KRAS or EGFR mutant lung cancer cells. Elevation of csGRP78 in the lung cancer cells was dependent on activation of the non-receptor tyrosine kinase SRC by CZT. Inhibition of SRC activation in the cancer cells prevented csGRP78 translocation but promoted Par-4 secretion by CZT, implying that activated SRC prevented Par-4 secretion. In normal cells, CZT did not activate SRC and csGRP78 elevation but induced Par-4 secretion. Consequently, CZT induced Par-4 secretion from normal cells and elevated csGRP78 in the ALK-negative tumor cells to cause paracrine apoptosis in cancer cell cultures and growth inhibition of tumor xenografts in mice. Thus, CZT induces differential activation of SRC in normal and cancer cells to trigger the pro-apoptotic Par-4-GRP78 axis. As csGRP78 is a targetable receptor, CZT can be repurposed to elevate csGRP78 for inhibition of ALK-negative lung tumors.
Keywords: Par-4; SRC; apoptosis; crizotinib; csGRP78; lung cancer.
AJCR Copyright © 2023.
Conflict of interest statement
VMR is owner of a start-up company Parcure, LLC, in Lexington, KY, USA. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Cell Surface GRP78 as a Death Receptor and an Anticancer Drug Target.Cancers (Basel). 2019 Nov 13;11(11):1787. doi: 10.3390/cancers11111787. Cancers (Basel). 2019. PMID: 31766302 Free PMC article.
-
Paracrine receptor activation by microenvironment triggers bypass survival signals and ALK inhibitor resistance in EML4-ALK lung cancer cells.Clin Cancer Res. 2012 Jul 1;18(13):3592-602. doi: 10.1158/1078-0432.CCR-11-2972. Epub 2012 May 2. Clin Cancer Res. 2012. PMID: 22553343
-
Cell surface GRP78 regulates TGFβ1-mediated profibrotic responses via TSP1 in diabetic kidney disease.Front Pharmacol. 2023 Feb 24;14:1098321. doi: 10.3389/fphar.2023.1098321. eCollection 2023. Front Pharmacol. 2023. PMID: 36909183 Free PMC article.
-
Will the Requirement by the US FDA to Simultaneously Co-Develop Companion Diagnostics (CDx) Delay the Approval of Receptor Tyrosine Kinase Inhibitors for RTK-Rearranged (ROS1-, RET-, AXL-, PDGFR-α-, NTRK1-) Non-Small Cell Lung Cancer Globally?Front Oncol. 2014 Apr 1;4:58. doi: 10.3389/fonc.2014.00058. eCollection 2014. Front Oncol. 2014. PMID: 24744988 Free PMC article. Review.
-
Linking cell-surface GRP78 to cancer: From basic research to clinical value of GRP78 antibodies.Cancer Lett. 2022 Jan 1;524:1-14. doi: 10.1016/j.canlet.2021.10.004. Epub 2021 Oct 9. Cancer Lett. 2022. PMID: 34637844 Review.
Cited by
-
Anaplastic lymphoma kinase inhibitors-a review of anticancer properties, clinical efficacy, and resistance mechanisms.Front Pharmacol. 2023 Oct 25;14:1285374. doi: 10.3389/fphar.2023.1285374. eCollection 2023. Front Pharmacol. 2023. PMID: 37954850 Free PMC article. Review.
-
UPF1 Alleviates Myocardial Ischemia-Reperfusion Injury by Regulating SMURF2-Mediated Ubiquitination Degradation of FOXA2.Korean Circ J. 2025 Apr;55(4):305-321. doi: 10.4070/kcj.2024.0190. Epub 2024 Dec 10. Korean Circ J. 2025. PMID: 39962965 Free PMC article.
References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
