Apolipoprotein M Attenuates Anthracycline Cardiotoxicity and Lysosomal Injury
- PMID: 37034289
- PMCID: PMC10077122
- DOI: 10.1016/j.jacbts.2022.09.010
Apolipoprotein M Attenuates Anthracycline Cardiotoxicity and Lysosomal Injury
Abstract
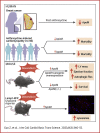
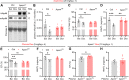
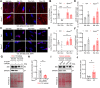
Apolipoprotein M (ApoM) binds sphingosine-1-phosphate (S1P) and is inversely associated with mortality in human heart failure (HF). Here, we show that anthracyclines such as doxorubicin (Dox) reduce circulating ApoM in mice and humans, that ApoM is inversely associated with mortality in patients with anthracycline-induced heart failure, and ApoM heterozygosity in mice increases Dox-induced mortality. In the setting of Dox stress, our studies suggest ApoM can help sustain myocardial autophagic flux in a post-transcriptional manner, attenuate Dox cardiotoxicity, and prevent lysosomal injury.
Keywords: TFEB; anthracycline; apolipoprotein M; autophagy; cardiomyopathy.
© 2023 The Authors.
Conflict of interest statement
Dr Javaheri was supported by R01HL155344 and K08HL138262 from the National Heart, Lung, and Blood Institute and by the Diabetes Research Center at Washington University in St Louis of the National Institutes of Health (NIH) under award number P30DK020579, as well as NIH grant P30DK056341 (Nutrition Obesity Research Center), and by the Children’s Discovery Institute of Washington University (MC-FR-2020-919) and St Louis Children’s Hospital. Dr Guo was supported by an American Heart Association Postdoctoral Fellowship (898679). Dr Diwan was supported by grants from the Department of Veterans Affairs (I01BX004235) and the NIH (HL107594, HL43431, and NS094692). Dr Scherrer-Crosbie is supported by R01HL130539 and R01HL131613. Dr Rawnsley was supported by training grant support from the NIH (T32007081). Dr Desai was supported by R01HL136603. Dr Bergom was supported by R01HL147884. Research reported in this publication was also supported by the National Cancer Institute of the NIH under award numbers R50CA211466 (Dr Rettig), R35CA210084 (Dr DiPersio), P01CA101937 (Dr DiPersio), and R01HL119962 (Dr Parks). Human heart tissue procurement was supported by the National Heart, Lung, and Blood Institute via R01HL105993 (Drs Margulies and Cappola). Drs Christoffersen and Hajny were supported by the Novo Nordisk Foundation (0053008 and NNF13OC0003898). Dr Stitziel was supported in part by R01HL131961, R01HL159171, P01HL151328, and UM1HG008853 and by the Foundation for Barnes-Jewish Hospital. We acknowledge support from the NIH Shared Instrumentation Grant (S10RR027552) for support through the Hope Center Neuroimaging Core, the Molecular Microbiology Imaging Facility, and the Advanced Imaging and Tissue Analysis Core of the Digestive Disease Research Core Center (DDRCC NIH P30DK052574) at Washington University School of Medicine. Dr Stitziel has received consulting fees from Sension Therapeutics and investigator-initiated research funding from Regeneron Pharmaceuticals unrelated to the content of this study. Dr Javaheri has a pending patent for fusion protein nanodiscs for the treatment of heart failure and eye diseases, receives research funding from AstraZeneca, and is on the Scientific Advisory Board of Mobius Scientific. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures





References
-
- Armenian S., Bhatia S. Predicting and preventing anthracycline-related cardiotoxicity. Am Soc Clin Oncol Educ Book. 2018;38:3–12. - PubMed
-
- Lipshultz S.E., Colan S.D., Gelber R.D., Perez-Atayde A.R., Sallan S.E., Sanders S.P. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808–815. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

