Synthesis of multi-module low density lipoprotein receptor class A domains with acid labile cyanopyridiniumylides (CyPY) as aspartic acid masking groups
- PMID: 37034404
- PMCID: PMC10074552
- DOI: 10.1039/d2cb00234e
Synthesis of multi-module low density lipoprotein receptor class A domains with acid labile cyanopyridiniumylides (CyPY) as aspartic acid masking groups
Abstract
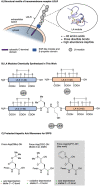
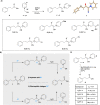
Low-density lipoprotein receptor class A domains (LA modules) are common ligand-binding domains of transmembrane receptors of approximately 40 amino acids that are involved in several cellular processes including endocytosis of extracellular targets. Due to their wide-ranging function and distribution among different transmembrane receptors, LA modules are of high interest for therapeutic applications. However, the efficient chemical synthesis of LA modules and derivatives is hindered by complications, many arising from the high abundance of aspartic acid and consequent aspartimide formation. Here, we report a robust, efficient and general applicable chemical synthesis route for accessing such LA modules, demonstrated by the synthesis and folding of the LA3 and LA4 modules of the low-density lipoprotein receptor, as well as a heterodimeric LA3-LA4 constructed by chemical ligation. The synthesis of the aspartic acid-rich LA domain peptides is made possible by the use of cyanopyridiniumylides (CyPY) - reported here for the first time - as a masking group for carboxylic acids. We show that cyanopyridiniumylide masked aspartic acid monomers are readily available building blocks for solid phase peptide synthesis and successfully suppress aspartimide formation. Unlike previously reported ylide-based carboxylic acid protecting groups, CyPY protected aspartic acids are converted to the free carboxylic acid by acidic hydrolysis and are compatible with all common residues and protecting groups. The chemical synthesis of Cys- and Asp-rich LA modules enables new access to a class of difficult to provide, but promising protein domains.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
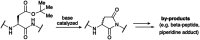


Similar articles
-
Prevention of aspartimide formation during peptide synthesis using cyanosulfurylides as carboxylic acid-protecting groups.Nat Commun. 2020 Feb 20;11(1):982. doi: 10.1038/s41467-020-14755-6. Nat Commun. 2020. PMID: 32080186 Free PMC article.
-
Preventing aspartimide formation in Fmoc SPPS of Asp-Gly containing peptides--practical aspects of new trialkylcarbinol based protecting groups.J Pept Sci. 2016 Feb;22(2):92-7. doi: 10.1002/psc.2844. Epub 2016 Jan 11. J Pept Sci. 2016. PMID: 26751703
-
Design of protecting groups for the beta-carboxylic group of aspartic acid that minimize base-catalyzed aspartimide formation.Int J Pept Protein Res. 1996 Oct;48(4):305-11. doi: 10.1111/j.1399-3011.1996.tb00846.x. Int J Pept Protein Res. 1996. PMID: 8919050
-
Microbiologically produced carboxylic acids used as building blocks in organic synthesis.Subcell Biochem. 2012;64:391-423. doi: 10.1007/978-94-007-5055-5_19. Subcell Biochem. 2012. PMID: 23080261 Review.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
Cited by
-
Chemical Synthesis and Chaperone Peptide Mediated Folding of Human Nerve Growth Factor by Expressed KAHA Ligation.ACS Cent Sci. 2025 May 1;11(8):1321-1328. doi: 10.1021/acscentsci.5c00277. eCollection 2025 Aug 27. ACS Cent Sci. 2025. PMID: 40893961 Free PMC article.
-
Identification, occurrence and prevention of aspartimide-related byproducts in chemical protein synthesis.Chem Sci. 2025 Jul 8;16(32):14496-14508. doi: 10.1039/d5sc03824c. eCollection 2025 Aug 13. Chem Sci. 2025. PMID: 40671757 Free PMC article.
References
LinkOut - more resources
Full Text Sources

