The relationship between epigenetic age and the hallmarks of aging in human cells
- PMID: 37034474
- PMCID: PMC10077971
- DOI: 10.1038/s43587-022-00220-0
The relationship between epigenetic age and the hallmarks of aging in human cells
Abstract
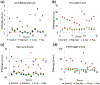
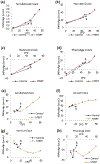
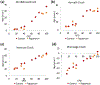
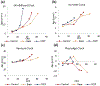
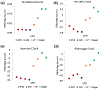
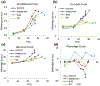
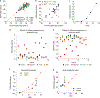
Epigenetic clocks are mathematically derived age estimators that are based on combinations of methylation values that change with age at specific CpGs in the genome. These clocks are widely used to measure the age of tissues and cells1,2. The discrepancy between epigenetic age (EpiAge), as estimated by these clocks, and chronological age is referred to as EpiAge acceleration. Epidemiological studies have linked EpiAge acceleration to a wide variety of pathologies, health states, lifestyle, mental state and environmental factors2, indicating that epigenetic clocks tap into critical biological processes that are involved in aging. Despite the importance of this inference, the mechanisms underpinning these clocks remained largely uncharacterized and unelucidated. Here, using primary human cells, we set out to investigate whether epigenetic aging is the manifestation of one or more of the aging hallmarks previously identified3. We show that although epigenetic aging is distinct from cellular senescence, telomere attrition and genomic instability, it is associated with nutrient sensing, mitochondrial activity and stem cell composition.
Conflict of interest statement
Competing interests S.H. is a cofounder of a nonprofit, the Epigenetic Clock Development Foundation, that serves to freely facilitate the use of these technology and methods. All other authors have no competing interests.
Figures







References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

