This is a preprint.
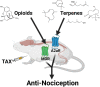
Terpenes from Cannabis sativa Induce Antinociception in Mouse Chronic Neuropathic Pain via Activation of Spinal Cord Adenosine A2A Receptors
- PMID: 37034662
- PMCID: PMC10081257
- DOI: 10.1101/2023.03.28.534594
Terpenes from Cannabis sativa Induce Antinociception in Mouse Chronic Neuropathic Pain via Activation of Spinal Cord Adenosine A2A Receptors
Update in
-
Terpenes from Cannabis sativa induce antinociception in a mouse model of chronic neuropathic pain via activation of adenosine A 2A receptors.Pain. 2024 Nov 1;165(11):e145-e161. doi: 10.1097/j.pain.0000000000003265. Epub 2024 May 2. Pain. 2024. PMID: 38709489 Free PMC article.
Abstract
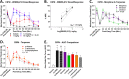
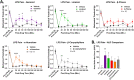
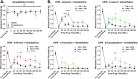
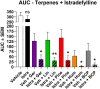
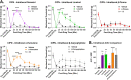
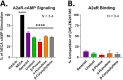
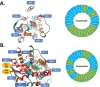
Terpenes are small hydrocarbon compounds that impart aroma and taste to many plants, including Cannabis sativa. A number of studies have shown that terpenes can produce pain relief in various pain states in both humans and animals. However, these studies were methodologically limited and few established mechanisms of action. In our previous work, we showed that the terpenes geraniol, linalool, β-pinene, α-humulene, and β-caryophyllene produced cannabimimetic behavioral effects via multiple receptor targets. We thus expanded this work to explore the efficacy and mechanism of these Cannabis terpenes in relieving chronic pain. We first tested for antinociceptive efficacy by injecting terpenes (200 mg/kg, IP) into male and female CD-1 mice with chemotherapy-induced peripheral neuropathy (CIPN) or lipopolysaccharide-induced inflammatory pain, finding that the terpenes produced roughly equal efficacy to 10 mg/kg morphine or 3.2 mg/kg WIN55,212. We further found that none of the terpenes produced reward as measured by conditioned place preference, while low doses of terpene (100 mg/kg) combined with morphine (3.2 mg/kg) produced enhanced antinociception vs. either alone. We then used the adenosine A2A receptor (A2AR) selective antagonist istradefylline (3.2 mg/kg, IP) and spinal cord-specific CRISPR knockdown of the A2AR to identify this receptor as the mechanism for terpene antinociception in CIPN. In vitro cAMP and binding studies and in silico modeling studies further suggested that the terpenes act as A2AR agonists. Together these studies identify Cannabis terpenes as potential therapeutics for chronic neuropathic pain, and identify a receptor mechanism in the spinal cord for this activity.
Keywords: Adenosine A2A Receptor; Analgesia; Cannabis; Neuropathic Pain; Terpenes.
Figures




References
-
- Yong R.J., Mullins P.M., and Bhattacharyya N., Prevalence of chronic pain among adults in the United States. Pain, 2022. 163(2): p. e328–e332. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
