This is a preprint.
Implantation of engineered adipocytes that outcompete tumors for resources suppresses cancer progression
- PMID: 37034710
- PMCID: PMC10081280
- DOI: 10.1101/2023.03.28.534564
Implantation of engineered adipocytes that outcompete tumors for resources suppresses cancer progression
Abstract
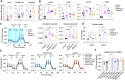
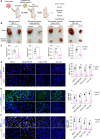
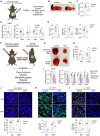
Tumors acquire an increased ability to obtain and metabolize nutrients. Here, we engineered and implanted adipocytes to outcompete tumors for nutrients and show that they can substantially reduce cancer progression. Growing cells or xenografts from several cancers (breast, colon, pancreas, prostate) alongside engineered human adipocytes or adipose organoids significantly suppresses cancer progression and reduces hypoxia and angiogenesis. Transplanting modulated adipocyte organoids in pancreatic or breast cancer mouse models nearby or distal from the tumor significantly suppresses its growth. To further showcase therapeutic potential, we demonstrate that co-culturing tumor organoids derived from human breast cancers with engineered patient-derived adipocytes significantly reduces cancer growth. Combined, our results introduce a novel cancer therapeutic approach, termed adipose modulation transplantation (AMT), that can be utilized for a broad range of cancers.
Conflict of interest statement
Competing interests NA is a cofounder and on the scientific advisory board of Regel Therapeutics and Neomer Diagnostics. NA receives funding from BioMarin Pharmaceutical Incorporate. HPN and NA have filed a patent application covering embodiments and concepts disclosed in the manuscript.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
