This is a preprint.
Altered muscle niche contributes to myogenic deficit in the D2- mdx model of severe DMD
- PMID: 37034785
- PMCID: PMC10081277
- DOI: 10.1101/2023.03.27.534413
Altered muscle niche contributes to myogenic deficit in the D2- mdx model of severe DMD
Update in
-
Altered muscle niche contributes to myogenic deficit in the D2-mdx model of severe DMD.Cell Death Discov. 2023 Jul 4;9(1):224. doi: 10.1038/s41420-023-01503-0. Cell Death Discov. 2023. PMID: 37402716 Free PMC article.
Abstract
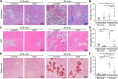
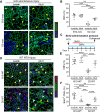
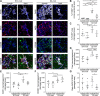
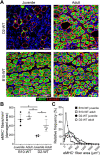
Lack of dystrophin is the genetic basis for the Duchenne muscular dystrophy (DMD). However, disease severity varies between patients, based on specific genetic modifiers. D2- mdx is a model for severe DMD that exhibits exacerbated muscle degeneration and failure to regenerate even in the juvenile stage of the disease. We show that poor regeneration of juvenile D2- mdx muscles is associated with enhanced inflammatory response to muscle damage that fails to resolve efficiently and supports excessive accumulation of fibroadipogenic progenitors (FAPs). Unexpectedly, the extent of damage and degeneration of juvenile D2- mdx muscle is reduced in adults and is associated with the restoration of the inflammatory and FAP responses to muscle injury. These improvements enhance myogenesis in the adult D2- mdx muscle, reaching levels comparable to the milder (B10- mdx ) mouse model of DMD. Ex vivo co-culture of healthy satellite cells (SCs) with the juvenile D2- mdx FAPs reduced their fusion efficacy and in vivo glucocorticoid treatment of juvenile D2 mouse improved muscle regeneration. Our findings indicate that aberrant stromal cell response contributes to poor myogenesis and greater muscle degeneration in dystrophic juvenile D2- mdx muscles and reversal of this reduces pathology in adult D2- mdx mouse muscle, identifying these as therapeutic targets to treat dystrophic DMD muscles.
Conflict of interest statement
Figures



References
-
- Hoffman EP, Brown RH Jr., Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51(6):919–28. - PubMed
-
- Mendell JR, Shilling C, Leslie ND, Flanigan KM, al-Dahhak R, Gastier-Foster J, et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Annals of neurology. 2012;71(3):304–13. - PubMed
-
- Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355(6362):696–702. - PubMed
-
- Allikian MJ, McNally EM. Processing and assembly of the dystrophin glycoprotein complex. Traffic. 2007;8(3):177–83. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
