Review of Source and Transportation Pathways of Perfluorinated Compounds Through the Air
- PMID: 37034823
- PMCID: PMC10078797
Review of Source and Transportation Pathways of Perfluorinated Compounds Through the Air
Abstract
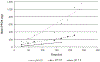
This article will identify the state of science on the generation, production, and transport of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA). Additionally, this article will focus on the transport of these environmental contaminants through air sources. It is important to explore why air exposure is critical to bring awareness to a problem that is not always immediately apparent. From a biological standpoint, clean air is necessary to sustain healthy life. Thus, it is key to understand the environmental transport of chemicals such as PFOS and PFOA with regard to their ability to migrate (i.e., air to water and water to air) and thus create unsafe air. The fluorinated backbone of these substances is both hydrophobic and oleophobic/lipophobic, while the terminal functional group is hydrophilic (water loving). Therefore, PFOS and PFOA compounds tend to partition to interfaces, such as between air and water with the fluorinated backbone residing in air and the terminal functional group residing in water. This article will identify opportunities for research to further the understanding of their potential impacts to human health.
Figures


References
-
- 3M Company. (2003). Environmental, health and safety measures relating to perfluorooctanoic acid and its salts (PFOA) (U.S. EPA EDocket OPPTS 2003–0012-0007). http://www.fluoridealert.org/wp-content/pesticides/effect.pfoa.class.mar...
-
- Abrahams ER, Kaste JM, Ouimet W, & Dethier DP (2018). Asymmetric hillslope erosion following wildfire in Fourmile Canyon, Colorado. Earth Surface Processes and Landforms, 43(9), 2009–2021.
-
- Anderson RH, Long GC, Porter RC, & Anderson JK (2016). Occurrence of select perfluoroalkyl substances at U.S. Air Force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere, 150, 678–685. - PubMed
-
- Barton CA, Butler LE, Zarzecki CJ, Flaherty J, & Kaiser M. (2006). Characterizing perfluorooctanoate in ambient air near the fence line of a manufacturing facility: Comparing modeled and monitored values. Journal of the Air and Waste Management Association, 56(1), 48–55. - PubMed
-
- Benskin JP, Yeung LWY, Yamashita N, Taniyasu S, Lam PKS, & Martin JW (2010). Perfluorinated acid isomer profiling in water and quantitative assessment of manufacturing source. Environmental Science & Technology, 44(23), 9049–9054. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
