[Health Technology Assessment: a value-based tool for the evaluation of healthcare technologies. Reassessment of the cell-culture-derived quadrivalent influenza vaccine: Flucelvax Tetra® 2.0]
- PMID: 37034835
- PMCID: PMC10079375
- DOI: 10.15167/2421-4248/jpmh2022.63.4s1
[Health Technology Assessment: a value-based tool for the evaluation of healthcare technologies. Reassessment of the cell-culture-derived quadrivalent influenza vaccine: Flucelvax Tetra® 2.0]
Figures



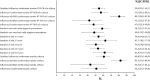
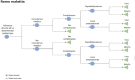
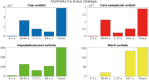
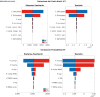


References
-
- EUnetHTA HTA definition. Available at: http://www.eunethta.eu/about-us/faq#t287n73 (accessed on 15/03/2022).
-
- Organizzazione Mondiale della Sanità (OMS). Health Technology Assessment. Available at: http://www.who.int/health-technology-assessment/about/healthtechnology/en/ (accessed on 15/0/2022).
-
- EUnetHTA. HTA Core Model Version 3.0. Available at: https://www.eunethta.eu/wp-content/uploads/2018/03/HTACoreModel3.0-1.pdf... (accessed on 15/03/2022).
-
- Velasco M, Perleth M, Drummond M, Gürtner F, Jørgensen T, Jovell A, Malone J, Rüther A, Wild C. Best practice in undertaking and reporting health technology assessments. Working group 4 report. Int J Technol Assess Health Care 2002;18:361-422. https://doi.org/10.1017/s0266462302000284 10.1017/s0266462302000284 - DOI - PubMed
-
- Soril LJ, MacKean G, Noseworthy TW, Leggett LE, Clement FM. Achieving optimal technology use: a proposed model for health technology reassessment. SAGE Open Med 2017;5:2050312117704861. https://doi.org/10.1177/2050312117704861 10.1177/2050312117704861 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
