Naturally occurring canine sarcomas: Bridging the gap from mouse models to human patients through cross-disciplinary research partnerships
- PMID: 37035209
- PMCID: PMC10076632
- DOI: 10.3389/fonc.2023.1130215
Naturally occurring canine sarcomas: Bridging the gap from mouse models to human patients through cross-disciplinary research partnerships
Abstract
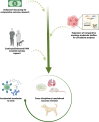
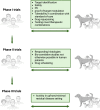
Fueled by support from the National Cancer Institute's "Cancer Moonshot" program, the past few years have witnessed a renewed interest in the canine spontaneous cancer model as an invaluable resource in translational oncology research. Increasingly, there is awareness that pet dogs with cancer provide an accessible bridge to improving the efficiency of cancer drug discovery and clinical therapeutic development. Canine tumors share many biological, genetic, and histologic features with their human tumor counterparts, and most importantly, retain the complexities of naturally occurring drug resistance, metastasis, and tumor-host immune interactions, all of which are difficult to recapitulate in induced or genetically engineered murine tumor models. The utility of canine models has been particularly apparent in sarcoma research, where the increased incidence of sarcomas in dogs as compared to people has facilitated comparative research resulting in treatment advances benefitting both species. Although there is an increasing awareness of the advantages in using spontaneous canine sarcoma models for research, these models remain underutilized, in part due to a lack of more permanent institutional and cross-institutional infrastructure to support partnerships between veterinary and human clinician-scientists. In this review, we provide an updated overview of historical and current applications of spontaneously occurring canine tumor models in sarcoma research, with particular attention to knowledge gaps, limitations, and growth opportunities within these applications. Furthermore, we propose considerations for working within existing veterinary translational and comparative oncology research infrastructures to maximize the benefit of partnerships between veterinary and human biomedical researchers within and across institutions to improve the utility and application of spontaneous canine sarcomas in translational oncology research.
Keywords: canine (dog); comparative oncology; immunotherapy; osteosarcoma; sarcoma.
Copyright © 2023 Klosowski, Haines, Alfino, McMellen, Leibowitz and Regan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Clinical, Pathological, and Ethical Considerations for the Conduct of Clinical Trials in Dogs with Naturally Occurring Cancer: A Comparative Approach to Accelerate Translational Drug Development.ILAR J. 2018 Dec 1;59(1):99-110. doi: 10.1093/ilar/ily019. ILAR J. 2018. PMID: 30668709 Free PMC article.
-
Canine sarcomas as a surrogate for the human disease.Pharmacol Ther. 2018 Aug;188:80-96. doi: 10.1016/j.pharmthera.2018.01.012. Epub 2018 Mar 9. Pharmacol Ther. 2018. PMID: 29378221 Free PMC article. Review.
-
Canine comparative oncology for translational radiation research.Int J Radiat Biol. 2022;98(3):496-505. doi: 10.1080/09553002.2021.1987572. Epub 2021 Oct 11. Int J Radiat Biol. 2022. PMID: 34586958 Review.
-
Naturally Occurring Canine Melanoma as a Predictive Comparative Oncology Model for Human Mucosal and Other Triple Wild-Type Melanomas.Int J Mol Sci. 2018 Jan 30;19(2):394. doi: 10.3390/ijms19020394. Int J Mol Sci. 2018. PMID: 29385676 Free PMC article. Review.
-
Developing a translational murine-to-canine pathway for an IL-2/agonist anti-CD40 antibody cancer immunotherapy.Vet Comp Oncol. 2022 Sep;20(3):602-612. doi: 10.1111/vco.12813. Epub 2022 Apr 6. Vet Comp Oncol. 2022. PMID: 35315197 Free PMC article.
Cited by
-
Prior corticosteroid treatment alters cPBMC composition and IFNγ response to immunotherapy in canine cancer.Front Immunol. 2025 Apr 24;16:1544949. doi: 10.3389/fimmu.2025.1544949. eCollection 2025. Front Immunol. 2025. PMID: 40342421 Free PMC article.
-
Cross-species evaluation of fibroblast activation protein alpha as potential imaging target for soft tissue sarcoma: a comparative immunohistochemical study in humans, dogs, and cats.Front Oncol. 2023 Sep 1;13:1210004. doi: 10.3389/fonc.2023.1210004. eCollection 2023. Front Oncol. 2023. PMID: 37727209 Free PMC article.
References
-
- Innovation or stagnation: Challenge and opportunity on the critical path to new medical products (2004) (US Food and Drug Administration Website: US Food and Drug Administration; - Critical Path Initiative). Available from: http://wayback.archiveit.org/7993/20180125032208/https://www.fda.gov/Sci....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

