Characterization of gut microbial and metabolite alterations in faeces of Goto Kakizaki rats using metagenomic and untargeted metabolomic approach
- PMID: 37035219
- PMCID: PMC10075032
- DOI: 10.4239/wjd.v14.i3.255
Characterization of gut microbial and metabolite alterations in faeces of Goto Kakizaki rats using metagenomic and untargeted metabolomic approach
Abstract
Background: In recent years, the incidence of type 2 diabetes (T2DM) has shown a rapid growth trend. Goto Kakizaki (GK) rats are a valuable model for the study of T2DM and share common glucose metabolism features with human T2DM patients. A series of studies have indicated that T2DM is associated with the gut microbiota composition and gut metabolites. We aimed to systematically characterize the faecal gut microbes and metabolites of GK rats and analyse the relationship between glucose and insulin resistance.
Aim: To evaluate the gut microbial and metabolite alterations in GK rat faeces based on metagenomics and untargeted metabolomics.
Methods: Ten GK rats (model group) and Wistar rats (control group) were observed for 10 wk, and various glucose-related indexes, mainly including weight, fasting blood glucose (FBG) and insulin levels, homeostasis model assessment of insulin resistance (HOMA-IR) and homeostasis model assessment of β cell (HOMA-β) were assessed. The faecal gut microbiota was sequenced by metagenomics, and faecal metabolites were analysed by untargeted metabolomics. Multiple metabolic pathways were evaluated based on the differential metabolites identified, and the correlations between blood glucose and the gut microbiota and metabolites were analysed.
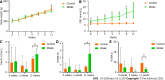
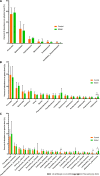
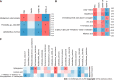
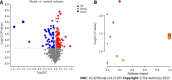
Results: The model group displayed significant differences in weight, FBG and insulin levels, HOMA-IR and HOMA-β indexes (P < 0.05, P < 0.01) and a shift in the gut microbiota structure compared with the control group. The results demonstrated significantly decreased abundances of Prevotella sp. CAG:604 and Lactobacillus murinus (P < 0.05) and a significantly increased abundance of Allobaculum stercoricanis (P < 0.01) in the model group. A correlation analysis indicated that FBG and HOMA-IR were positively correlated with Allobaculum stercoricanis and negatively correlated with Lactobacillus murinus. An orthogonal partial least squares discriminant analysis suggested that the faecal metabolic profiles differed between the model and control groups. Fourteen potential metabolic biomarkers, including glycochenodeoxycholic acid, uric acid, 13(S)-hydroxyoctadecadienoic acid (HODE), N-acetylaspartate, β-sitostenone, sphinganine, 4-pyridoxic acid, and linoleic acid, were identified. Moreover, FBG and HOMA-IR were found to be positively correlated with glutathione, 13(S)-HODE, uric acid, 4-pyridoxic acid and allantoic acid and ne-gatively correlated with 3-α, 7-α, chenodeoxycholic acid glycine conjugate and 26-trihydroxy-5-β-cholestane (P < 0.05, P < 0.01). Allobaculum stercoricanis was positively correlated with linoleic acid and sphinganine (P < 0.01), and 2-methyl-3-hydroxy-5-formylpyridine-4-carboxylate was negatively associated with Prevotella sp. CAG:604 (P < 0.01). The metabolic pathways showing the largest differences were arginine biosynthesis; primary bile acid biosynthesis; purine metabolism; linoleic acid metabolism; alanine, aspartate and glutamate metabolism; and nitrogen metabolism.
Conclusion: Metagenomics and untargeted metabolomics indicated that disordered compositions of gut microbes and metabolites may be common defects in GK rats.
Keywords: Goto-Kakizaki rats; Gut microbial; Metabolites; Metagenomics; Type 2 diabetes mellitus; Untargeted metabolomics.
©The Author(s) 2023. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no conflicts of interest.
Figures


Similar articles
-
Effect of berberine on hyperglycaemia and gut microbiota composition in type 2 diabetic Goto-Kakizaki rats.World J Gastroenterol. 2021 Feb 28;27(8):708-724. doi: 10.3748/wjg.v27.i8.708. World J Gastroenterol. 2021. PMID: 33716449 Free PMC article.
-
Integrated 16S rRNA Sequencing, Metagenomics, and Metabolomics to Characterize Gut Microbial Composition, Function, and Fecal Metabolic Phenotype in Non-obese Type 2 Diabetic Goto-Kakizaki Rats.Front Microbiol. 2020 Jan 20;10:3141. doi: 10.3389/fmicb.2019.03141. eCollection 2019. Front Microbiol. 2020. PMID: 32038574 Free PMC article.
-
Metagenomics and Faecal Metabolomics Integrative Analysis towards the Impaired Glucose Regulation and Type 2 Diabetes in Uyghur-Related Omics.J Diabetes Res. 2019 Nov 18;2019:2893041. doi: 10.1155/2019/2893041. eCollection 2019. J Diabetes Res. 2019. PMID: 31828159 Free PMC article.
-
Effect of traditional Chinese medicine on gut microbiota in adults with type 2 diabetes: A systematic review and meta-analysis.Phytomedicine. 2021 Jul 15;88:153455. doi: 10.1016/j.phymed.2020.153455. Epub 2020 Dec 30. Phytomedicine. 2021. PMID: 33478831
-
A short review on the features of the non-obese diabetic Goto-Kakizaki rat intestine.Braz J Med Biol Res. 2022 Aug 22;55:e11910. doi: 10.1590/1414-431X2022e11910. eCollection 2022. Braz J Med Biol Res. 2022. PMID: 36000611 Free PMC article. Review.
Cited by
-
Proteomic Analysis of the Effects of Shenzhu Tiaopi Granules on Model Rats with Type 2 Diabetes Mellitus.Diabetes Metab Syndr Obes. 2025 Feb 25;18:583-599. doi: 10.2147/DMSO.S493036. eCollection 2025. Diabetes Metab Syndr Obes. 2025. PMID: 40026899 Free PMC article.
-
Linear association of the dietary index for gut microbiota with insulin resistance and type 2 diabetes mellitus in U.S. adults: the mediating role of body mass index and inflammatory markers.Front Nutr. 2025 Mar 21;12:1557280. doi: 10.3389/fnut.2025.1557280. eCollection 2025. Front Nutr. 2025. PMID: 40191795 Free PMC article.
References
-
- Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H, Shi B, Sun H, Ba J, Chen B, Du J, He L, Lai X, Li Y, Chi H, Liao E, Liu C, Liu L, Tang X, Tong N, Wang G, Zhang JA, Wang Y, Xue Y, Yan L, Yang J, Yang L, Yao Y, Ye Z, Zhang Q, Zhang L, Zhu J, Zhu M, Ning G, Mu Y, Zhao J, Teng W, Shan Z. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ . 2020;369:m997. - PMC - PubMed
-
- Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia . 2003;46:3–19. - PubMed
-
- Nagao M, Esguerra JLS, Wendt A, Asai A, Sugihara H, Oikawa S, Eliasson L. Selectively Bred Diabetes Models: GK Rats, NSY Mice, and ON Mice. Methods Mol Biol. 2020;2128:25–54. - PubMed
-
- Zhang Q, Hong Z, Zhu J, Zeng C, Tang Z, Wang W, Huang H. Biliopancreatic Limb Length of Small Intestinal Bypass in Non-obese Goto-Kakizaki (GK) Rats Correlates with Gastrointestinal Hormones, Adipokines, and Improvement in Type 2 Diabetes. Obes Surg . 2021;31:4419–4426. - PubMed
-
- Portha B. Programmed disorders of beta-cell development and function as one cause for type 2 diabetes? Diabetes Metab Res Rev . 2005;21:495–504. - PubMed
LinkOut - more resources
Full Text Sources

