Nε-(carboxymethyl)lysine promotes lipid uptake of macrophage via cluster of differentiation 36 and receptor for advanced glycation end products
- PMID: 37035231
- PMCID: PMC10075039
- DOI: 10.4239/wjd.v14.i3.222
Nε-(carboxymethyl)lysine promotes lipid uptake of macrophage via cluster of differentiation 36 and receptor for advanced glycation end products
Abstract
Background: Advanced glycation end products (AGEs) are diabetic metabolic toxic products that cannot be ignored. Nε-(carboxymethyl)lysine (CML), a component of AGEs, could increase macrophage lipid uptake, promote foam cell formation, and thereby accelerate atherosclerosis. The receptor for AGEs (RAGE) and cluster of differentiation 36 (CD36) were the receptors of CML. However, it is still unknown whether RAGE and CD36 play key roles in CML-promoted lipid uptake.
Aim: Our study aimed to explore the role of RAGE and CD36 in CML-induced mac-rophage lipid uptake.
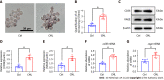
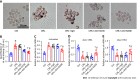
Methods: In this study, we examined the effect of CML on lipid uptake by Raw264.7 macrophages. After adding 10 mmol/L CML, the lipid accumulation in macro-phages was confirmed by oil red O staining. Expression changes of CD36 and RAGE were detected with immunoblotting and quantitative real-time polymerase chain reaction. The interaction between CML with CD36 and RAGE was verified by immunoprecipitation. We synthesized a novel N-succinimidyl-4-18F-fluorobenzoate-CML radioactive probe. Radioactive receptor-ligand binding assays were performed to test the binding affinity between CML with CD36 and RAGE. The effects of blocking CD36 or RAGE on CML-promoting lipid uptake were also detected.
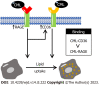
Results: The study revealed that CML significantly promoted lipid uptake by macro-phages. Immunoprecipitation and radioactive receptor-ligand binding assays indicated that CML could specifically bind to both CD36 and RAGE. CML had a higher affinity for CD36 than RAGE. ARG82, ASN71, and THR70 were the potential interacting amino acids that CD36 binds to CML Anti-CD36 and anti-RAGE could block the uptake of CML by macrophages. The lipid uptake promotion effect of CML was significantly attenuated after blocking CD36 or RAGE.
Conclusion: Our results suggest that the binding of CML with CD36 and RAGE promotes macrophage lipid uptake.
Keywords: Cluster of differentiation 36; Lipid uptake; Macrophage; Nε-(carboxymethyl)lysine; Receptor for advanced glycation end products.
©The Author(s) 2023. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures


References
-
- Yamagishi S, Matsui T, Ueda S, Nakamura K, Imaizumi T. Advanced glycation end products (AGEs) and cardiovascular disease (CVD) in diabetes. Cardiovasc Hematol Agents Med Chem. 2007;5:236–240. - PubMed
-
- Wang ZQ, Jing LL, Yan JC, Sun Z, Bao ZY, Shao C, Pang QW, Geng Y, Zhang LL, Li LH. Role of AGEs in the progression and regression of atherosclerotic plaques. Glycoconj J. 2018;35:443–450. - PubMed
-
- Schalkwijk CG, Micali LR, Wouters K. Advanced glycation endproducts in diabetes-related macrovascular complications: focus on methylglyoxal. Trends Endocrinol Metab. 2023;34:49–60. - PubMed
LinkOut - more resources
Full Text Sources

