N-Arylacetamide derivatives of methyl 1,2-benzothiazine-3-carboxylate as potential drug candidates for urease inhibition
- PMID: 37035287
- PMCID: PMC10073911
- DOI: 10.1098/rsos.230104
N-Arylacetamide derivatives of methyl 1,2-benzothiazine-3-carboxylate as potential drug candidates for urease inhibition
Abstract
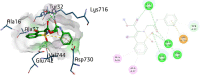
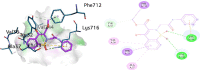
Urease enzyme is an infectious factor that provokes the growth and colonization of virulence pathogenic bacteria in humans. To overcome the deleterious effects of bacterial infections, inhibition of urease enzyme is one of the promising approaches. The current study is designed to synthesize new 1,2-benzothiazine-N-arylacetamide derivatives 5(a-n) that can effectively provide a new drug candidate to avoid bacterial infections by urease inhibition. After structural elucidation by FT-IR, proton and carbon-13 NMR and mass spectroscopy, the synthesized compounds 5(a-n) were investigated to evaluate their inhibitory potential against urease enzyme. In vitro analysis against positive control of thiourea indicated that all the synthesized compounds have strong inhibitory strengths as compared to the reference drug. Compound 5k, being the most potent inhibitor, strongly inhibited the urease enzymes and revealed an IC50 value of 9.8 ± 0.023 µM when compared with the IC50 of thiourea (22.3 ± 0.031 µM)-a far more robust inhibitory potential. Docking studies of 5k within the urease active site revealed various significant interactions such as H-bond, π-alkyl with amino acid residues like Val744, Lys716, Ala16, Glu7452, Ala37 and Asp730.
Keywords: benzothiazine; docking; synthesis; thiourea; urease; ureolytic.
© 2023 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures



Similar articles
-
Exploration of morpholine-thiophene hybrid thiosemicarbazones for the treatment of ureolytic bacterial infections via targeting urease enzyme: Synthesis, biochemical screening and computational analysis.Front Chem. 2024 May 24;12:1403127. doi: 10.3389/fchem.2024.1403127. eCollection 2024. Front Chem. 2024. PMID: 38855062 Free PMC article.
-
Design, synthesis, and biological studies of the new cysteine-N-arylacetamide derivatives as a potent urease inhibitor.Naunyn Schmiedebergs Arch Pharmacol. 2024 Jan;397(1):305-315. doi: 10.1007/s00210-023-02596-1. Epub 2023 Jul 12. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 37436497
-
Novel thiobarbiturates as potent urease inhibitors with potential antibacterial activity: Design, synthesis, radiolabeling and biodistribution study.Bioorg Med Chem. 2020 Dec 1;28(23):115759. doi: 10.1016/j.bmc.2020.115759. Epub 2020 Sep 12. Bioorg Med Chem. 2020. PMID: 32992246
-
Three New Acrylic Acid Derivatives from Achillea mellifolium as Potential Inhibitors of Urease from Jack Bean and α-Glucosidase from Saccharomyces cerevisiae.Molecules. 2022 Aug 6;27(15):5004. doi: 10.3390/molecules27155004. Molecules. 2022. PMID: 35956953 Free PMC article.
-
New acetylphenol-based acyl thioureas broaden the scope of drug candidates for urease inhibition: synthesis, in vitro screening and in silico analysis.Int J Biol Macromol. 2022 Feb 15;198:157-167. doi: 10.1016/j.ijbiomac.2021.12.064. Epub 2021 Dec 22. Int J Biol Macromol. 2022. PMID: 34953808
Cited by
-
Pyridylpiperazine-based carbodithioates as urease inhibitors: synthesis and biological evaluation.Front Chem. 2024 Aug 6;12:1423385. doi: 10.3389/fchem.2024.1423385. eCollection 2024. Front Chem. 2024. PMID: 39165334 Free PMC article.
-
Exploration of morpholine-thiophene hybrid thiosemicarbazones for the treatment of ureolytic bacterial infections via targeting urease enzyme: Synthesis, biochemical screening and computational analysis.Front Chem. 2024 May 24;12:1403127. doi: 10.3389/fchem.2024.1403127. eCollection 2024. Front Chem. 2024. PMID: 38855062 Free PMC article.
-
Synthesis, characterization, antimicrobial, cytotoxic and carbonic anhydrase inhibition activities of multifunctional pyrazolo-1,2-benzothiazine acetamides.Beilstein J Org Chem. 2025 Feb 12;21:348-357. doi: 10.3762/bjoc.21.25. eCollection 2025. Beilstein J Org Chem. 2025. PMID: 39968288 Free PMC article.
References
-
- Karita M, Tsuda M, Nakazawa T. 1995. Essential role of urease in vitro and in vivo Helicobacter pylori colonization study using a wild-type and isogenic urease mutant strain. J. Clin. Gastroenterol. 21, S160-S163. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

