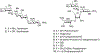Synthesis of 4- O-(4-Amino-4-deoxy-β-D-xylopyranosyl)paromomycin and 4- S-(β-D-Xylopyranosyl)-4-deoxy-4'-thio-paromomycin and Evaluation of their Antiribosomal and Antibacterial Activity
- PMID: 37035443
- PMCID: PMC10081503
- DOI: 10.1016/j.tet.2023.133330
Synthesis of 4- O-(4-Amino-4-deoxy-β-D-xylopyranosyl)paromomycin and 4- S-(β-D-Xylopyranosyl)-4-deoxy-4'-thio-paromomycin and Evaluation of their Antiribosomal and Antibacterial Activity
Abstract
The design, synthesis and antiribosomal and antibacterial activity of two novel glycosides of the aminoglycoside antibiotic paromomycin are described. The first carries of 4-amino-4-deoxy-β-D-xylopyranosyl moiety at the paromomycin 4'-position and is approximately two-fold more active than the corresponding β-D-xylopyranosyl derivative. The second is a 4'-(β-D-xylopyranosylthio) derivative of 4'-deoxyparomomycin that is unexpectedly less active than the simple β-D-xylopyranosyl derivative, perhaps because of the insertion of the conformationally more mobile thioglycosidic linkage.
Conflict of interest statement
Declaration of Competing Interest. AV, SNH and DC are cofounders of and retain an equity position in Juvabis AG, a biotech start-up developing next generation aminoglycoside antibiotics. All other authors declare no competing interests. Declaration of interests The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Armstrong ES, Kostrub CF, Cass RT, Moser HE, Serio AW and Miller GH, in Antibiotic Discovery and Development, eds. Dougherty TJ and Pucci MJ, Springer Science+Business Media, New York, 2012, pp. 229–269.
-
- Takahashi Y and Igarashi M, J. Antibiotics, 2018, 71, 4–14.
-
- Magnet S and Blanchard JS, Chem. Rev, 2005, 105, 477–497. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources