Photosensitized co-generation of nitric oxide and singlet oxygen Enhanced toxicity against ovarian cancer cells
- PMID: 37035485
- PMCID: PMC10081534
- DOI: 10.1007/s11051-022-05463-x
Photosensitized co-generation of nitric oxide and singlet oxygen Enhanced toxicity against ovarian cancer cells
Abstract
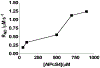
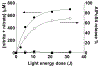
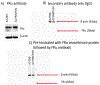
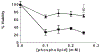
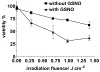
Near micromolar concentrations of nitric oxide (NO) induce tumor cells death. However, an appropriate NO load has to be delivered selectively to the tumor site in order to avoid NO loss and secondary NO-induced effects. The encapsulation of millimolar concentrations of a NO source and an appropriate trigger of NO release within phospatidylcholine-based liposomes should provide an efficient tool for the selective release of the needed NO payload. In this work we report the photosensitized generation of singlet oxygen and NO from folate-targeted PEGylated liposomes, containing AlPcS4 as the sensitizer and S-nitrosoglutathione (GSNO), in millimolar amounts, as the NO source. Amounts of singlet oxygen detected outside the liposome when using PEGylated liposomes are near 200 % larger when GSNO is present inside the liposomes as compared to its absence. These liposomes, conjugated to folate, were found to enhance the photosensitized cytotoxicity to A2780CP20 ovarian cancer cells as compared to liposomes containing the sensitizer but no GSNO (30 % as compared to 70 % cell viability) under the conditions of this work. Fluorescense of AlPcS4 was observed inside cells incubated with folate-conjugated liposomes but not with liposomes without folate. The photosensitized activity enhancement by GSNO increased when light fluence or liposome concentration were increased. The majority of ovarian cancer patients are initially diagnosed with disseminated intra-abdominal disease (stages III-IV) and have a 5-year survival of less than 20%. This work suggests a novel ovarian cancer nodules treatment via the use of tumor-targeted liposome nanoparticles with the capability of generating simultaneously reactive oxygen and nitrogen species upon illumination with near-infrared light.
Conflict of interest statement
Conflicts of Interest: The authors declare no conflict of interest. The funding sources had no role in study design, data collection, analysis and interpretation or in writing and submitting the manuscript.
Figures
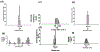

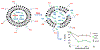
References
-
- Shen Z, Ma Q, Zhou X, Zhang G, Hao G, Sun Y et al. (2021) Strategies to improve photodynamic therapy efficacy by relieving the tumor hypoxia environment. NPG Asia Mater, 13:1–19.
-
- Di Nicola M, Williams BK Jr, Hua J, Bekerman VP, Mashayekhi A, Shields JA et al. (2021) Photodynamic Therapy (PDT) for Retinal Hemangioblastoma: Treatment Outcomes in 17 Consecutive Patients. Ophthalmol Retina,. - PubMed
-
- Rak J, Pouckova P, Benes J, Vetvicka D (2019) Drug delivery systems for phthalocyanines for photodynamic therapy. Anticancer Res 39:3323–39. - PubMed
-
- Shi Z, Meng X, Zhang K, Tang S, Zhang C, Yang Z et al. (2021) Engineering Structural Metal–Organic Framework for Hypoxia-Tolerant Type I Photodynamic Therapy against Hypoxic Cancer. ACS Mater Lett 3:781–9.
-
- Nwogu C, Kloc A, Attwood K, Bshara W, Durrani F, Pandey R (2021) Porfimer Sodium Versus PS785 for Photodynamic Therapy (PDT) of Lung Cancer Xenografts in Mice (A Novel Agent for Lung Cancer PDT). J Surg Res 263:245–50. - PubMed
