An abiotic, tetrameric, eight-helix bundle
- PMID: 37035688
- PMCID: PMC10074428
- DOI: 10.1039/d3sc00267e
An abiotic, tetrameric, eight-helix bundle
Abstract
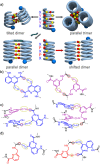
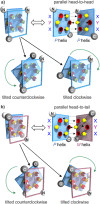
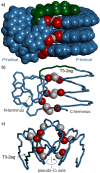
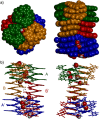
Four helically folded aromatic oligoamide sequences containing either a chiral monomer based on 2-(2-aminophenoxy)-propionic acid, an N-terminal (1H)-camphanyl group, or both, were synthesized. Spectroscopic solution investigations using 1H NMR and circular dichroism (CD) demonstrated that the 2-(2-aminophenoxy)-propionic acid unit biases helix handedness quantitatively in chloroform and dichloromethane. It even quantitatively overcomes an opposing effect of the camphanyl group and thus ensures reliable helix handedness control. A series of nine sequences composed of two helically folded aromatic oligoamide segments separated by a flexible linker based on a di-, tri- or tetraethylene glycol unit were then synthesized. In these sequences, helix handedness was controlled by means of an N-terminal (1H)-camphanyl group or a 2-(2-aminophenoxy)-propionic acid units in either both helical segments, or only in the N-terminal segment, or in none of the segments. The helical segments all displayed hydroxy and carbonyl groups at their surfaces as hydrogen bond donors and acceptors so as to promote helix-to-helix hydrogen bonding. NMR and CD spectroscopic studies showed that, in some cases, well-defined, stable, discrete abiotic helix-turn-helix tertiary folds form in organic solvents. Molecular modelling suggests that these correspond to structures in which the two helix axes are at an angle. In one case, the absence of handedness control resulted in a complex and large aggregate. A solid state structure obtained by single crystal X-ray diffraction analysis revealed a tetrameric assembly composed of eight helices with both right and left handedness arranged in three subdomains consisting of two hydrogen-bonded three-helix bundles and one two-helix-bundle. Several helix-to-helix hydrogen bonds were mediated by bridging water molecules. This structure constitutes an important milestone in the construction of abiotic protein-like architectures.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Computational Prediction and Rationalization, and Experimental Validation of Handedness Induction in Helical Aromatic Oligoamide Foldamers.Chemistry. 2017 Mar 13;23(15):3605-3615. doi: 10.1002/chem.201605082. Epub 2017 Jan 16. Chemistry. 2017. PMID: 27935190
-
Absolute control of helical handedness in quinoline oligoamides.J Org Chem. 2011 Jan 7;76(1):195-200. doi: 10.1021/jo1019442. Epub 2010 Dec 13. J Org Chem. 2011. PMID: 21142109
-
Homochiral versus Heterochiral Dimeric Helical Foldamer Bundles: Chlorinated-Solvent-Dependent Self-Sorting.Angew Chem Int Ed Engl. 2023 Mar 13;62(12):e202217325. doi: 10.1002/anie.202217325. Epub 2023 Feb 8. Angew Chem Int Ed Engl. 2023. PMID: 36625790
-
Domain Swapping in Abiotic Foldamers.Angew Chem Int Ed Engl. 2024 Jul 8;63(28):e202405091. doi: 10.1002/anie.202405091. Epub 2024 Jun 4. Angew Chem Int Ed Engl. 2024. PMID: 38661252
-
Absolute handedness control of oligoamide double helices by chiral oxazolylaniline induction.Org Biomol Chem. 2020 Sep 14;18(34):6643-6650. doi: 10.1039/d0ob01503b. Epub 2020 Aug 21. Org Biomol Chem. 2020. PMID: 32821892
Cited by
-
Design of an abiotic unimolecular three-helix bundle.Chem Sci. 2024 Dec 4;16(3):1136-1146. doi: 10.1039/d4sc07336c. eCollection 2025 Jan 15. Chem Sci. 2024. PMID: 39640026 Free PMC article.
References
LinkOut - more resources
Full Text Sources

