Amides as modifiable directing groups in electrophilic borylation
- PMID: 37035693
- PMCID: PMC10074396
- DOI: 10.1039/d2sc06483a
Amides as modifiable directing groups in electrophilic borylation
Abstract
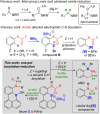
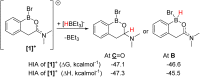
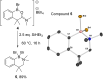
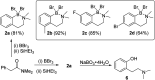
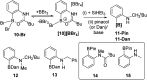
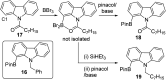
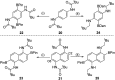
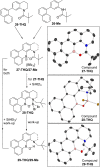
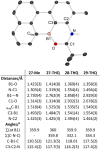
Amide directed C-H borylation using ≥two equiv. of BBr3 forms borenium cations containing a R2N(R')C[double bond, length as m-dash]O→B(Ar)Br unit which has significant Lewis acidity at the carbonyl carbon. This enables reduction of the amide unit to an amine using hydrosilanes. This approach can be applied sequentially in a one-pot electrophilic borylation-reduction process, which for phenyl-acetylamides generates ortho borylated compounds that can be directly oxidised to the 2-(2-aminoethyl)-phenol. Other substrates amenable to the C-H borylation-reduction sequence include mono and diamino-arenes and carbazoles. This represents a simple method to make borylated molecules that would be convoluted to access otherwise (e.g. N-octyl-1-BPin-carbazole). Substituent variation is tolerated at boron as well as in the amide unit, with diarylborenium cations also amenable to reduction. This enables a double C-H borylation-reduction-hydrolysis sequence to access B,N-polycyclic aromatic hydrocarbons (PAHs), including an example where both the boron and nitrogen centres contain functionalisable handles (N-H and B-OH). This method is therefore a useful addition to the metal-free borylation toolbox for accessing useful intermediates (ArylBPin) and novel B,N-PAHs.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



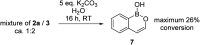
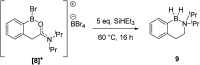

References
-
-
For select recent reviews on directed C–H borylation see:
- Iqbal S. A. Pahl J. Yuan K. Ingleson M. J. Chem. Soc. Rev. 2020;49:4564. doi: 10.1039/C9CS00763F. - DOI - PubMed
- Ros A. Fernández R. Lassaletta J. M. Chem. Soc. Rev. 2014;43:3229. doi: 10.1039/C3CS60418G. - DOI - PubMed
- Mihai M. T. Genov G. R. Phipps R. J. Chem. Soc. Rev. 2018;47:149. doi: 10.1039/C7CS00637C. - DOI - PubMed
-
-
- Boronic Acids: Preparation and Applications, ed. D. Hall, Wiley-VCH, Weinheim, 2011
-
-
For select reviews on iridium catalysed borylation see:
- Mkhalid I. A. I. Barnard J. H. Marder T. B. Murphy J. M. Hartwig J. F. Chem. Rev. 2010;110:890. doi: 10.1021/cr900206p. - DOI - PubMed
- Bisht R. Haldar C. Hassan M. M. M. Hoque M. E. Chaturvedi J. Chattopadhyay B. Chem. Soc. Rev. 2022;51:5042. doi: 10.1039/D1CS01012C. - DOI - PubMed
-
-
-
For recent reviews on electrophilic C–H borylation see:
- Hazra S. Mahato S. Das K. K. Panda S. Chem.–Eur. J. 2022:e202200556. - PubMed
- Rej S. Chatani N. Angew. Chem., Int. Ed. 2022;61:e202209539. doi: 10.1002/anie.202209539. - DOI - PubMed
- Tan X. Wang H. Chem. Soc. Rev. 2022;51:2583. doi: 10.1039/D2CS00044J. - DOI - PubMed
-
. For select very recent publications in this area see:
- Berionni G. Angew. Chem., Int. Ed. 2022;61:e202210284. doi: 10.1002/anie.202210284. - DOI - PubMed
- Xu X. Wu G. Yang Z. Liu X. Hao L. Wang Y. Ma Z. Ji Y. Org. Lett. 2022;24:7163. doi: 10.1021/acs.orglett.2c02858. - DOI - PubMed
- Pahl J. Noone E. Uzelac M. Yuan K. Ingleson M. J. Angew. Chem., Int. Ed. 2022;61:e202206230. doi: 10.1002/anie.202206230. - DOI - PMC - PubMed
- Lei K. Jiang Z. Lalancette R. A. Tang X. Jäkle F. J. Am. Chem. Soc. 2022;144:18908. doi: 10.1021/jacs.2c06538. - DOI - PubMed
- Sadek O. Le Gac A. Hidalgo N. Mallet-Ladeira S. Miqueu K. Bouhadir G. Bourissou D. Angew. Chem., Int. Ed. 2022;61:e202110102. doi: 10.1002/anie.202110102. - DOI - PubMed
-
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

