Enabling the formation of native mAb, Fab' and Fc-conjugates using a bis-disulfide bridging reagent to achieve tunable payload-to-antibody ratios (PARs)
- PMID: 37035695
- PMCID: PMC10074397
- DOI: 10.1039/d2sc06318b
Enabling the formation of native mAb, Fab' and Fc-conjugates using a bis-disulfide bridging reagent to achieve tunable payload-to-antibody ratios (PARs)
Abstract
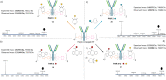
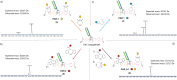
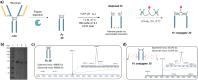
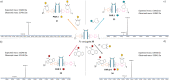
Either as full IgGs or as fragments (Fabs, Fc, etc.), antibodies have received tremendous attention in the development of new therapeutics such as antibody-drug conjugates (ADCs). The production of ADCs involves the grafting of active payloads onto an antibody, which is generally enabled by the site-selective modification of native or engineered antibodies via chemical or enzymatic methods. Whatever method is employed, controlling the payload-antibody ratio (PAR) is a challenge in terms of multiple aspects including: (i) obtaining homogeneous protein conjugates; (ii) obtaining unusual PARs (PAR is rarely other than 2, 4 or 8); (iii) using a single method to access a range of different PARs; (iv) applicability to various antibody formats; and (v) flexibility for the production of heterofunctional antibody-conjugates (e.g. attachment of multiple types of payloads). In this article, we report a single pyridazinedione-based trifunctional dual bridging linker that enables, in a two-step procedure (re-bridging/click), the generation of either mAb-, Fab'-, or Fc-conjugates from native mAb, (Fab')2 or Fc formats, respectively. Fc and (Fab')2 formats were generated via enzymatic digestion of native mAbs. Whilst the same reduction and re-bridging protocols were applied to all three of the protein formats, the subsequent click reaction(s) employed to graft payload(s) drove the generation of a range of PARs, including heterofunctional PARs. As such, exploiting click reactivity and/or orthogonality afforded mAb-conjugates with PARs of 6, 4, 2 or 4 + 2, and Fab'- and Fc-conjugates with a PAR of 3, 2, 1 or 2 + 1 on-demand. We believe that the homogeneity, novelty and variety in accessible PARs, as well as the applicability to various antibody-conjugate formats enabled by our non-recombinant method could be a suitable tool for antibody-drug conjugates optimisation (optimal PAR value, optimal payloads combination) and boost the development of new antibody therapeutics (Fab'- and Fc-conjugates).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
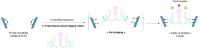



Similar articles
-
Evaluation of Cellular Targeting by Fab' vs Full-Length Antibodies in Antibody-Nanoparticle Conjugates (ANCs) Using CD4 T-cells.Bioconjug Chem. 2022 Mar 16;33(3):486-495. doi: 10.1021/acs.bioconjchem.2c00024. Epub 2022 Feb 9. Bioconjug Chem. 2022. PMID: 35139308 Free PMC article.
-
Characterization of Disulfide Bond Rebridged Fab-Drug Conjugates Prepared Using a Dual Maleimide Pyrrolobenzodiazepine Cytotoxic Payload.ChemMedChem. 2019 Jun 18;14(12):1185-1195. doi: 10.1002/cmdc.201900077. Epub 2019 May 3. ChemMedChem. 2019. PMID: 30980702
-
Binding parameters and idiotypic profile of the whole immunoglobulin and Fab' fragments of murine monoclonal antibody to distinct determinants of the human high molecular weight-melanoma associated antigen.Cancer Res. 1992 May 1;52(9):2497-503. Cancer Res. 1992. PMID: 1373670
-
Recent Chemical Approaches for Site-Specific Conjugation of Native Antibodies: Technologies toward Next-Generation Antibody-Drug Conjugates.Chembiochem. 2019 Nov 4;20(21):2729-2737. doi: 10.1002/cbic.201900178. Epub 2019 Jul 17. Chembiochem. 2019. PMID: 30973187 Review.
-
Full-length recombinant antibodies from Escherichia coli: production, characterization, effector function (Fc) engineering, and clinical evaluation.MAbs. 2022 Jan-Dec;14(1):2111748. doi: 10.1080/19420862.2022.2111748. MAbs. 2022. PMID: 36018829 Free PMC article. Review.
Cited by
-
Antibody-drug conjugates in cancer therapy: innovations, challenges, and future directions.Arch Pharm Res. 2024 Jan;47(1):40-65. doi: 10.1007/s12272-023-01479-6. Epub 2023 Dec 28. Arch Pharm Res. 2024. PMID: 38153656 Review.
-
Site-directed conjugation of single-stranded DNA to affinity proteins: quantifying the importance of conjugation strategy.Chem Sci. 2024 May 7;15(23):8982-8992. doi: 10.1039/d4sc01838a. eCollection 2024 Jun 12. Chem Sci. 2024. PMID: 38873052 Free PMC article.
-
Induction, growth, drug resistance, and metastasis: A comprehensive summary of the relationship between STAT3 and gastric cancer.Heliyon. 2024 Sep 4;10(18):e37263. doi: 10.1016/j.heliyon.2024.e37263. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39309860 Free PMC article. Review.
-
Modular Semisynthetic Approach to Generate T Cell-Dependent Bispecific Constructs from Recombinant IgG1 Antibodies.Bioconjug Chem. 2024 Sep 16;35(10):1524-31. doi: 10.1021/acs.bioconjchem.4c00309. Online ahead of print. Bioconjug Chem. 2024. PMID: 39284580 Free PMC article.
-
A platform for SpyCatcher conjugation to native antibodies.Chem Sci. 2025 May 6;16(23):10602-10609. doi: 10.1039/d5sc02286j. eCollection 2025 Jun 11. Chem Sci. 2025. PMID: 40386161 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

