Rational design of a genetically encoded NMR zinc sensor
- PMID: 37035699
- PMCID: PMC10074429
- DOI: 10.1039/d3sc00437f
Rational design of a genetically encoded NMR zinc sensor
Abstract
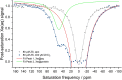
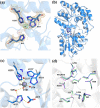
Elucidating the biochemical roles of the essential metal ion, Zn2+, motivates detection strategies that are sensitive, selective, quantitative, and minimally invasive in living systems. Fluorescent probes have identified Zn2+ in cells but complementary approaches employing nuclear magnetic resonance (NMR) are lacking. Recent studies of maltose binding protein (MBP) using ultrasensitive 129Xe NMR spectroscopy identified a switchable salt bridge which causes slow xenon exchange and elicits strong hyperpolarized 129Xe chemical exchange saturation transfer (hyper-CEST) NMR contrast. To engineer the first genetically encoded, NMR-active sensor for Zn2+, we converted the MBP salt bridge into a Zn2+ binding site, while preserving the specific xenon binding cavity. The zinc sensor (ZS) at only 1 μM achieved 'turn-on' detection of Zn2+ with pronounced hyper-CEST contrast. This made it possible to determine different Zn2+ levels in a biological fluid via hyper-CEST. ZS was responsive to low-micromolar Zn2+, only modestly responsive to Cu2+, and nonresponsive to other biologically important metal ions, according to hyper-CEST NMR spectroscopy and isothermal titration calorimetry (ITC). Protein X-ray crystallography confirmed the identity of the bound Zn2+ ion using anomalous scattering: Zn2+ was coordinated with two histidine side chains and three water molecules. Penta-coordinate Zn2+ forms a hydrogen-bond-mediated gate that controls the Xe exchange rate. Metal ion binding affinity, 129Xe NMR chemical shift, and exchange rate are tunable parameters via protein engineering, which highlights the potential to develop proteins as selective metal ion sensors for NMR spectroscopy and imaging.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Programming xenon diffusion in maltose-binding protein.Biophys J. 2022 Dec 6;121(23):4635-4643. doi: 10.1016/j.bpj.2022.10.025. Epub 2022 Oct 20. Biophys J. 2022. PMID: 36271622 Free PMC article.
-
An Expanded Palette of Xenon-129 NMR Biosensors.Acc Chem Res. 2016 Oct 18;49(10):2179-2187. doi: 10.1021/acs.accounts.6b00309. Epub 2016 Sep 19. Acc Chem Res. 2016. PMID: 27643815 Free PMC article.
-
Bacterial spore detection and analysis using hyperpolarized 129Xe chemical exchange saturation transfer (Hyper-CEST) NMR.Chem Sci. 2014 Aug 1;5(8):3197-3203. doi: 10.1039/c4sc01190b. Chem Sci. 2014. PMID: 25089181 Free PMC article.
-
Molecular Sensing with Host Systems for Hyperpolarized 129Xe.Molecules. 2020 Oct 11;25(20):4627. doi: 10.3390/molecules25204627. Molecules. 2020. PMID: 33050669 Free PMC article. Review.
-
Analyzing free zinc(II) ion concentrations in cell biology with fluorescent chelating molecules.Metallomics. 2015 Feb;7(2):202-11. doi: 10.1039/c4mt00230j. Metallomics. 2015. PMID: 25362967 Review.
Cited by
-
Hyperpolarized Xenon-129 Chemical Exchange Saturation Transfer (HyperCEST) Molecular Imaging: Achievements and Future Challenges.Int J Mol Sci. 2024 Feb 5;25(3):1939. doi: 10.3390/ijms25031939. Int J Mol Sci. 2024. PMID: 38339217 Free PMC article. Review.
-
The role of responsive MRI probes in the past and the future of molecular imaging.Chem Sci. 2024 Nov 27;15(48):20122-20154. doi: 10.1039/d4sc04849k. eCollection 2024 Dec 11. Chem Sci. 2024. PMID: 39611034 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

