Photophysics of the red-form Kaede chromophore
- PMID: 37035701
- PMCID: PMC10074405
- DOI: 10.1039/d3sc00368j
Photophysics of the red-form Kaede chromophore
Abstract
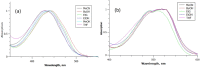
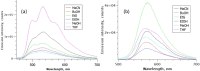
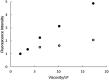
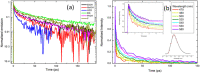
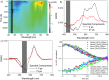
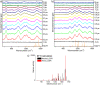
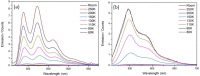
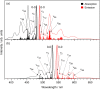
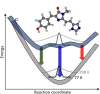
The green fluorescent protein (GFP) drove revolutionary progress in bioimaging. Photoconvertible fluorescent proteins (PCFPs) are an important branch of the FP family, of which Kaede is the prototype. Uniquely, PCFPs can be permanently switched from green to red emitting forms on UV irradiation, facilitating applications in site-specific photolabelling and protein tracking. Optimisation and exploitation of FPs requires understanding of the photophysical and photochemical behaviour of the chromophore. Accordingly, the principal GFP chromophore has been the subject of intense experimental and theoretical investigation. In contrast, the photophysics of the red emitting PCFP chromophore are largely unstudied. Here we present a detailed investigation of the excited-state properties of the Kaede chromophore in solution, utilising steady state measurements, ultrafast time-resolved electronic and vibrational spectroscopies, and electronic structure theory. Its excited state dynamics are very different to those of the parent GFP. Most remarkably, the PCFP chromophore has highly complex wavelength-dependent fluorescence decays and a mean lifetime an order of magnitude longer than the GFP chromophore. Transient electronic and vibrational spectroscopies suggest that these dynamics arise from a range of excited-state conformers that are spectrally and kinetically distinct but chemically similar. These conformers are populated directly by excitation of a broad thermal distribution of ground state structures about a single conformer, suggesting an excited-state potential surface with several minima. Temperature-dependence confirms the existence of barriers on the excited-state surface and reveals the radiationless decay mechanism to be internal conversion. These experimental observations are consistent with a model assuming a simple ground state potential energy surface accessing a complex excited state possessing multiple minima.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures
References
-
- Miyawaki A. Sawano A. Kogure T. Lighting up cells: labelling proteins with fluorophores. Nat. Rev. Mol. Cell Biol. 2003:S1–S7. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

