ebony affects pigmentation divergence and cuticular hydrocarbons in Drosophila americana and D. novamexicana
- PMID: 37035752
- PMCID: PMC10077920
- DOI: 10.3389/fevo.2020.00184
ebony affects pigmentation divergence and cuticular hydrocarbons in Drosophila americana and D. novamexicana
Abstract
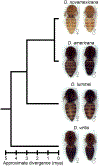
Drosophila pigmentation has been a fruitful model system for understanding the genetic and developmental mechanisms underlying phenotypic evolution. For example, prior work has shown that divergence of the tan gene contributes to pigmentation differences between two members of the virilis group: Drosophila novamexicana, which has a light yellow body color, and D. americana, which has a dark brown body color. Quantitative trait locus (QTL) mapping and expression analysis has suggested that divergence of the ebony gene might also contribute to pigmentation differences between these two species. Here, we directly test this hypothesis by using CRISPR/Cas9 genome editing to generate ebony null mutants in D. americana and D. novamexicana and then using reciprocal hemizygosity testing to compare the effects of each species' ebony allele on pigmentation. We find that divergence of ebony does indeed contribute to the pigmentation divergence between species, with effects on both the overall body color as well as a difference in pigmentation along the dorsal abdominal midline. Motivated by recent work in D. melanogaster, we also used the ebony null mutants to test for effects of ebony on cuticular hydrocarbon (CHC) profiles. We found that ebony affects CHC abundance in both species, but does not contribute to qualitative differences in the CHC profiles between these two species. Additional transgenic resources for working with D. americana and D. novamexicana, such as white mutants of both species and yellow mutants in D. novamexicana, were generated in the course of this work and are also described. Taken together, this study advances our understanding of loci contributing to phenotypic divergence and illustrates how the latest genome editing tools can be used for functional testing in non-model species.
Keywords: CRISPR; Cas9; abdominal pigmentation; genome editing; melanin; nanos; virilis group.
Conflict of interest statement
8Conflict of Interest Statement The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Bartelt RJ, Armold MT, Schaner AM, and Jackson LL (1986). Comparative analysis of cuticular hydrocarbons in the Drosophila virilis species group. Comp. Biochem. Physiol. -- Part B Biochem 83, 731–42. doi: 10.1016/0305-0491(86)90138-0. - DOI
-
- Benjamini Y and Hochberg Y (1995). Controlling the false discovery rate : a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300. https://www.jstor.org/stable/2346101.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
