circRNA_17725 Promotes Macrophage Polarization towards M2 by Targeting FAM46C to Alleviate Arthritis
- PMID: 37035757
- PMCID: PMC10081909
- DOI: 10.1155/2023/6818524
circRNA_17725 Promotes Macrophage Polarization towards M2 by Targeting FAM46C to Alleviate Arthritis
Abstract
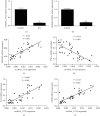
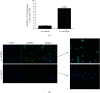
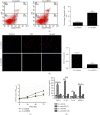
Accumulating studies have implicated that circular RNAs (circRNAs) play vital roles in the pathogenesis of rheumatoid arthritis (RA). Dysregulation of macrophage polarization leads to immune homeostatic imbalance in RA. However, the altering effects and mechanisms of circRNAs on macrophages polarization and immune homeostatic balance remain largely unclear. We aimed to investigate the potential role of circRNA_17725 in RA. The high-throughput sequence was performed to identify the dysregulated circRNAs in RA. We confirmed the data by CCK-8, EdU, and Annexin V/PI staining to elucidate the proliferation and apoptosis. The expressions of M1/M2-associated markers were confirmed using real-time PCR and flow cytometry analysis. Luciferase reporter assay and RNA Binding Protein Immunoprecipitation (RIP) were used to demonstrate the underlying mechanism of circRNA_17725. The altering effect of circRNA_17725 on macrophages in vivo was evaluated using collagen-induced arthritis (CIA) mouse model. circRNA_17725 was demonstrated to be downregulated in peripheral blood mononuclear cells and CD14+ monocytes from RA cases in contrast to healthy controls. The negative association between circRNA_17725 and the disease activity indexes (CRP, ESR, and DAS28) was observed, suggesting a vital role of circRNA_17725 in RA disease activity. Besides, after a coexpression analysis based on high-input sequencing and the bioinformatics analysis in MiRanda and TargetScan databases, a circRNA_17725-miR-4668-5p-FAM46C competing endogenous RNA (ceRNA) network was hypothesized. A series of cytology experiments in vitro have implicated that circRNA_17725 could inhibit the proliferation but enhance the apoptosis of macrophages. Decreased expression of TNF-α, IL-1β, and MMP-9 were observed in the supernatant of circRNA_17725-overexpressed Raw264.7 macrophages, implicating the inhibitory effect of circRNA_17725 on macrophage inflammatory mediators. Furthermore, circRNA_17725 could promote macrophage polarization towards M2 by targeting miR-4668-5p/FAM46C as a miRNA sponge. Additionally, circRNA_17725-overexpressed macrophages alleviated arthritis and protected against joint injuries and bone destruction by inducing macrophage polarization towards M2 in collagen-induced arthritis (CIA) mice. This study has suggested that circRNA_17725 regulated macrophage proliferation, apoptosis, inflammation, and polarization by sponging miR-4668-5p and upregulating FAM46C in RA.
Copyright © 2023 Chunjuan Yang et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






Similar articles
-
CircRNA_09505 aggravates inflammation and joint damage in collagen-induced arthritis mice via miR-6089/AKT1/NF-κB axis.Cell Death Dis. 2020 Oct 7;11(10):833. doi: 10.1038/s41419-020-03038-z. Cell Death Dis. 2020. PMID: 33028811 Free PMC article.
-
Circular RNA circ_0130438 suppresses TNF-α-induced proliferation, migration, invasion and inflammation in human fibroblast-like MH7A synoviocytes by regulating miR-130a-3p/KLF9 axis.Transpl Immunol. 2022 Jun;72:101588. doi: 10.1016/j.trim.2022.101588. Epub 2022 Mar 28. Transpl Immunol. 2022. PMID: 35358709
-
Circular RNA circ_0008360 Inhibits the Proliferation, Migration, and Inflammation and Promotes Apoptosis of Fibroblast-Like Synoviocytes by Regulating miR-135b-5p/HDAC4 Axis in Rheumatoid Arthritis.Inflammation. 2022 Feb;45(1):196-211. doi: 10.1007/s10753-021-01538-4. Epub 2021 Aug 30. Inflammation. 2022. PMID: 34462830
-
Dysregulation of circRNAs in rheumatoid arthritis, with special emphasis on circRNAs secreted by exosomes and the crosstalk between circRNAs and RNA methylations.Int Immunopharmacol. 2023 Sep;122:110549. doi: 10.1016/j.intimp.2023.110549. Epub 2023 Jul 6. Int Immunopharmacol. 2023. PMID: 37421778 Review.
-
Regulation of autophagy by circular RNAs in rheumatoid arthritis: Potential targets of action.Int J Rheum Dis. 2023 May;26(5):831-840. doi: 10.1111/1756-185X.14665. Epub 2023 Mar 30. Int J Rheum Dis. 2023. PMID: 36997810 Review.
Cited by
-
Exploration of potential shared gene signatures between periodontitis and multiple sclerosis.BMC Oral Health. 2024 Jan 13;24(1):75. doi: 10.1186/s12903-023-03846-7. BMC Oral Health. 2024. PMID: 38218802 Free PMC article.
-
Exosomal Osteoclast-Derived miRNA in Rheumatoid Arthritis: From Their Pathogenesis in Bone Erosion to New Therapeutic Approaches.Int J Mol Sci. 2024 Jan 25;25(3):1506. doi: 10.3390/ijms25031506. Int J Mol Sci. 2024. PMID: 38338785 Free PMC article. Review.
-
Dissecting the Puzzling Roles of FAM46C: A Multifaceted Pan-Cancer Tumour Suppressor with Increasing Clinical Relevance.Cancers (Basel). 2024 Apr 27;16(9):1706. doi: 10.3390/cancers16091706. Cancers (Basel). 2024. PMID: 38730656 Free PMC article. Review.
-
In Rheumatoid Arthritis, A Review of ncRNAs Related to NF-κB Signaling Pathways.Curr Pharm Biotechnol. 2025;26(3):319-327. doi: 10.2174/0113892010262829240214061103. Curr Pharm Biotechnol. 2025. PMID: 38424418 Review.
-
Circular RNAs in human diseases.MedComm (2020). 2024 Sep 4;5(9):e699. doi: 10.1002/mco2.699. eCollection 2024 Sep. MedComm (2020). 2024. PMID: 39239069 Free PMC article. Review.
References
-
- Giannini D., Antonucci M., Petrelli F., Bilia S., Alunno A., Puxeddu I. One year in review 2020: pathogenesis of rheumatoid arthritis. Clinical and Experimental Rheumatology . 2020;38(3):387–397. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

