Anti-Fibrotic Effects of DL-Glyceraldehyde in Hepatic Stellate Cells via Activation of ERK-JNK-Caspase-3 Signaling Axis
- PMID: 37035877
- PMCID: PMC10315341
- DOI: 10.4062/biomolther.2022.131
Anti-Fibrotic Effects of DL-Glyceraldehyde in Hepatic Stellate Cells via Activation of ERK-JNK-Caspase-3 Signaling Axis
Abstract
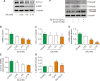
During liver injury, hepatic stellate cells can differentiate into myofibroblast-like structures, which are more susceptible to proliferation, migration, and extracellular matrix generation, leading to liver fibrosis. Anaerobic glycolysis is associated with activated stellate cells and glyceraldehyde (GA) is an inhibitor of glucose metabolism. Therefore, this study aimed to investigate the anti-fibrotic effects of GA in human stellate LX-2 cells. In this study, we used cell viability, morphological analysis, fluorescence-activated cell sorting (FACS), western blotting, and qRT-PCR techniques to elucidate the molecular mechanism underlying the anti-fibrotic effects of GA in LX-2 cells. The results showed that GA significantly reduced cell density and inhibited cell proliferation and lactate levels in LX-2 cells but not in Hep-G2 cells. We found that GA prominently increased the activation of caspase-3/9 for apoptosis induction, and a pan-caspase inhibitor, Z-VAD-fmk, attenuated the cell death and apoptosis effects of GA, suggesting caspase-dependent cell death. Moreover, GA strongly elevated reactive oxygen species (ROS) production and notably increased the phosphorylation of ERK and JNK. Interestingly, it dramatically reduced α-SMA and collagen type I protein and mRNA expression levels in LX-2 cells. Thus, inhibition of ERK and JNK activation significantly rescued GA-induced cell growth suppression and apoptosis in LX-2 cells. Collectively, the current study provides important information demonstrating the anti-fibrotic effects of GA, a glycolytic metabolite, and demonstrates the therapeutic potency of metabolic factors in liver fibrosis.
Keywords: Apoptosis; Collagen type-I; DL-glyceraldehyde; Hepatic stellate cells; MAPKs; α-SMA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Potentiation of hepatic stellate cell activation by extracellular ATP is dependent on P2X7R-mediated NLRP3 inflammasome activation.Pharmacol Res. 2017 Mar;117:82-93. doi: 10.1016/j.phrs.2016.11.040. Epub 2016 Dec 8. Pharmacol Res. 2017. PMID: 27940204
-
The Bioactive Extract of Pinnigorgia sp. Induces Apoptosis of Hepatic Stellate Cells via ROS-ERK/JNK-Caspase-3 Signaling.Mar Drugs. 2018 Jan 9;16(1):19. doi: 10.3390/md16010019. Mar Drugs. 2018. PMID: 29315209 Free PMC article.
-
Wedelolactone exhibits anti-fibrotic effects on human hepatic stellate cell line LX-2.Eur J Pharmacol. 2013 Aug 15;714(1-3):105-11. doi: 10.1016/j.ejphar.2013.06.012. Epub 2013 Jun 18. Eur J Pharmacol. 2013. PMID: 23791612
-
Endoplasmic reticulum stress-induced hepatic stellate cell apoptosis through calcium-mediated JNK/P38 MAPK and Calpain/Caspase-12 pathways.Mol Cell Biochem. 2014 Sep;394(1-2):1-12. doi: 10.1007/s11010-014-2073-8. Epub 2014 Jun 25. Mol Cell Biochem. 2014. PMID: 24961950
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
Cited by
-
New Insights into AMPK, as a Potential Therapeutic Target in Metabolic Dysfunction-Associated Steatotic Liver Disease and Hepatic Fibrosis.Biomol Ther (Seoul). 2025 Jan 1;33(1):18-38. doi: 10.4062/biomolther.2024.188. Epub 2024 Dec 20. Biomol Ther (Seoul). 2025. PMID: 39702310 Free PMC article. Review.
-
Anti-Fibrotic Effect of Oleamide Identified from the Moringa oleifera Lam. Leaves via Inhibition of TGF-β1-Induced SMAD2/3 Signaling Pathway.Int J Mol Sci. 2025 Apr 4;26(7):3388. doi: 10.3390/ijms26073388. Int J Mol Sci. 2025. PMID: 40244247 Free PMC article.
References
-
- Chen Y., Choi S. S., Michelotti G. A., Chan I. S., Swiderska-Syn M., Karaca G. F., Xie G., Moylan C. A., Garibaldi F., Premont R., Suliman H. B., Piantadosi C. A., Diehl A. M. Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology. 2012;143:1319–1329.e11. doi: 10.1053/j.gastro.2012.07.115. - DOI - PMC - PubMed
-
- Cortes E., Lachowski D., Rice A., Chronopoulos A., Robinson B., Thorpe S., Lee D. A., Possamai L. A., Wang H., Pinato D. J., Del Río Hernández A. E. Retinoic acid receptor-β is downregulated in hepatocellular carcinoma and cirrhosis and its expression inhibits myosin-driven activation and durotaxis in hepatic stellate cells. Hepatology. 2019;69:785–802. doi: 10.1002/hep.30193. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

