Super-Elastic Carbonized Mushroom Aerogel for Management of Uncontrolled Hemorrhage
- PMID: 37035946
- PMCID: PMC10238221
- DOI: 10.1002/advs.202207347
Super-Elastic Carbonized Mushroom Aerogel for Management of Uncontrolled Hemorrhage
Abstract
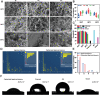
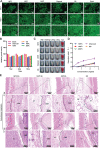
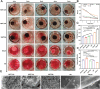
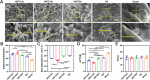
Uncontrolled hemorrhage is still the most common cause of potentially preventable death after trauma in prehospital settings. However, there rarely are hemostatic materials that can achieve safely and efficiently rapid hemostasis simultaneously. Here, new carbonized cellulose-based aerogel hemostatic material is developed for the management of noncompressible torso hemorrhage, the most intractable issue of uncontrolled hemorrhage. The carbonized cellulose aerogel is derived from the Agaricus bisporus after a series of processing, including cutting, carbonization, purification, and freeze-drying. In vitro, the carbonized cellulose aerogels with porous structure show improved hydrophilicity, good blood absorption, and coagulation ability, rapid shape recoverable ability under wet conditions. And in vivo, the carbonized aerogels show effective hemostatic ability in both small and big animal serious hemorrhage models. The amount of blood loss and the hemostatic time of carbonized aerogels are all better than the positive control group. Moreover, the mechanism studies reveal that the good hemostatic ability of the carbonized cellulose aerogel is associated with high hemoglobin binding efficiency, red blood cell absorption, and platelets absorption and activation. Together, the carbonized aerogel developed in this study could be promising for the management of uncontrolled hemorrhage.
Keywords: cellulose; hemostatic materials; platelet activation; porous structure; uncontrolled hemorrhage.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Carbonized Cellulose Aerogel Derived from Waste Pomelo Peel for Rapid Hemostasis of Trauma-Induced Bleeding.Adv Sci (Weinh). 2024 May;11(19):e2307409. doi: 10.1002/advs.202307409. Epub 2024 Mar 13. Adv Sci (Weinh). 2024. PMID: 38477567 Free PMC article.
-
Superhydrophobic Cellulose Nanofiber Aerogels for Efficient Hemostasis with Minimal Blood Loss.ACS Appl Mater Interfaces. 2024 Sep 11;16(36):47294-47302. doi: 10.1021/acsami.4c10505. Epub 2024 Sep 1. ACS Appl Mater Interfaces. 2024. PMID: 39219058
-
Engineering High-Performance Composite Cellulose Materials for Fast Hemostasis.ACS Biomater Sci Eng. 2024 Aug 12;10(8):5313-5326. doi: 10.1021/acsbiomaterials.4c01045. Epub 2024 Jul 25. ACS Biomater Sci Eng. 2024. PMID: 39051461
-
Chitosan-based hemostatic sponges as new generation hemostatic materials for uncontrolled bleeding emergency: Modification, composition, and applications.Carbohydr Polym. 2023 Jul 1;311:120780. doi: 10.1016/j.carbpol.2023.120780. Epub 2023 Mar 4. Carbohydr Polym. 2023. PMID: 37028883 Review.
-
Research status and development potential of composite hemostatic materials.J Mater Chem B. 2020 Jul 1;8(25):5395-5410. doi: 10.1039/d0tb00906g. J Mater Chem B. 2020. PMID: 32494795 Review.
Cited by
-
Dragon's Blood-Loaded Mesoporous Silica Nanoparticles for Rapid Hemostasis and Antibacterial Activity.Molecules. 2024 Apr 21;29(8):1888. doi: 10.3390/molecules29081888. Molecules. 2024. PMID: 38675708 Free PMC article.
-
Carbonized Cellulose Aerogel Derived from Waste Pomelo Peel for Rapid Hemostasis of Trauma-Induced Bleeding.Adv Sci (Weinh). 2024 May;11(19):e2307409. doi: 10.1002/advs.202307409. Epub 2024 Mar 13. Adv Sci (Weinh). 2024. PMID: 38477567 Free PMC article.
-
In Vitro Hemostatic Activity of Novel Fish Gelatin-Alginate Sponge (FGAS) Prototype.Polymers (Basel). 2024 Jul 18;16(14):2047. doi: 10.3390/polym16142047. Polymers (Basel). 2024. PMID: 39065364 Free PMC article.
-
Injectable and rapidly expandable thrombin-decorated cryogels achieve rapid hemostasis and high survival rates in a swine model of lethal junctional hemorrhage.Bioact Mater. 2024 Apr 30;38:154-168. doi: 10.1016/j.bioactmat.2024.04.024. eCollection 2024 Aug. Bioact Mater. 2024. PMID: 38721595 Free PMC article.
-
A self-elastic chitosan sponge integrating active and passive hemostatic mechanisms for effectively managing uncontrolled coagulopathic hemorrhage.Mater Today Bio. 2024 Mar 23;26:101031. doi: 10.1016/j.mtbio.2024.101031. eCollection 2024 Jun. Mater Today Bio. 2024. PMID: 38558772 Free PMC article.
References
-
- Morrison J. J., Crit. Care Clin. 2017, 33, 37. - PubMed
-
- Morrison J. J., Stannard A., Rasmussen T. E., Jansen J. O., Tai N. R. M., Midwinter M. J., J. Trauma Acute Care Surg. 2013, 75, S263. - PubMed
-
- Rago A. P., Duggan M. J., Hannett P., Brennecke L. H., LaRochelle A., Khatri C., Zugates G. T., Chang Y., Sharma U., King D. R., J. Trauma Acute Care Surg. 2015, 79, S78. - PubMed
-
- Leclerc S., Doshi S., Rezende‐Neto J. B., J. Mil. 2022, 8, 29.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
