Single-epitope T cell-based vaccine protects against SARS-CoV-2 infection in a preclinical animal model
- PMID: 37036004
- PMCID: PMC10132166
- DOI: 10.1172/jci.insight.167306
Single-epitope T cell-based vaccine protects against SARS-CoV-2 infection in a preclinical animal model
Abstract
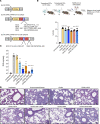
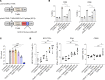
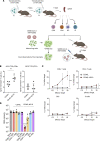
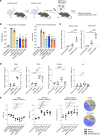
Currently authorized COVID-19 vaccines induce humoral and cellular responses to epitopes in the SARS-CoV-2 spike protein, though the relative roles of antibodies and T cells in protection are not well understood. To understand the role of vaccine-elicited T cell responses in protection, we established a T cell-only vaccine using a DC-targeted lentiviral vector expressing single CD8+ T cell epitopes of the viral nucleocapsid, spike, and ORF1. Immunization of angiotensin-converting enzyme 2-transgenic mice with ex vivo lentiviral vector-transduced DCs or by direct injection of the vector induced the proliferation of functional antigen-specific CD8+ T cells, resulting in a 3-log decrease in virus load upon live virus challenge that was effective against the ancestral virus and Omicron variants. The Pfizer/BNT162b2 vaccine was also protective in mice, but the antibodies elicited did not cross-react on the Omicron variants, suggesting that the protection was mediated by T cells. The studies suggest that the T cell response plays an important role in vaccine protection. The findings suggest that the incorporation of additional T cell epitopes into current vaccines would increase their effectiveness and broaden protection.
Keywords: COVID-19; Dendritic cells; Immunology; T cells.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

