Increased GS-II lectin binding and SATB2 downregulation are biological features for sessile serrated lesions and microvesicular hyperplastic polyps
- PMID: 37036163
- PMCID: PMC11551811
- DOI: 10.1111/pin.13321
Increased GS-II lectin binding and SATB2 downregulation are biological features for sessile serrated lesions and microvesicular hyperplastic polyps
Abstract
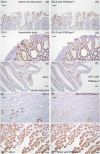
Sessile serrated lesions (SSLs) and microvesicular hyperplastic polyps (MVHPs) are colorectal lesions displaying gastric differentiation. Griffonia simplicifolia-II (GS-II) is a lectin specific to terminal α/βGlcNAc residues. Here, we assessed GS-II binding and performed immunostaining for HIK1083 (specific to terminal αGlcNAc residues), MUC5AC, MUC6, and special AT-rich sequence binding protein 2 (SATB2) in SSLs, MVHPs, and tubular adenomas (TAs). We observed MUC5AC positivity in 28 of 30 SSLs, but in only three of 23 TAs. Moreover, 24 of 30 SSLs were MUC6-positive, while none of the 23 TAs were MUC6-positive. None of the 30 SSLs or 23 TAs showed HIK1083 positivity. All 30 SSLs and 26 MVHPs were GS-II-positive, while only seven of 23 were in TAs. GS-II staining was mainly distributed in the Golgi region, but SSLs and MVHPs showed goblet cell distribution, in 20 of 30 and 19 of 26 cases, respectively. All SSLs, MVHPs, and TAs were SATB2-positive, but 21 of 30 SSLs and 12 of 26 MVHPs showed decreased staining intensity relative to adjacent mucosa, a decrease seen in only two of 23 in TAs. These results indicate overall that increased terminal βGlcNAc and decreased SATB2 expression are characteristics of SSLs and MVHPs.
Keywords: GS-II; SATB2; microvesicular hyperplastic polyps; sessile serrated lesions; βGlcNAc.
© 2023 The Authors. Pathology International published by Japanese Society of Pathology and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Characteristics of Clinically Significant Hyperplastic Polyps: Distinctions Between Microvesicular and Goblet Cell-Rich Types.J Gastroenterol Hepatol. 2025 May;40(5):1182-1187. doi: 10.1111/jgh.16921. Epub 2025 Mar 2. J Gastroenterol Hepatol. 2025. PMID: 40025862
-
Endoscopic characteristics to differentiate SSLs and microvesicular hyperplastic polyps from goblet cell-rich hyperplastic polyps.Endosc Int Open. 2024 Nov 7;12(11):E1251-E1259. doi: 10.1055/a-2301-6463. eCollection 2024 Nov. Endosc Int Open. 2024. PMID: 39524198 Free PMC article.
-
Epidemiology of goblet cell and microvesicular hyperplastic polyps.Am J Gastroenterol. 2014 Dec;109(12):1922-32. doi: 10.1038/ajg.2014.325. Epub 2014 Oct 28. Am J Gastroenterol. 2014. PMID: 25350766
-
Mucin 5AC as a Biomarker for Sessile Serrated Lesions: Results From a Systematic Review and Meta-Analysis.Clin Transl Gastroenterol. 2025 May 1;16(5):e00831. doi: 10.14309/ctg.0000000000000831. Clin Transl Gastroenterol. 2025. PMID: 40110854 Free PMC article.
-
Microvesicular hyperplastic polyp and sessile serrated lesion of the large intestine: a biological continuum or separate entities?J Clin Pathol. 2023 Jul;76(7):429-434. doi: 10.1136/jcp-2023-208783. Epub 2023 Mar 16. J Clin Pathol. 2023. PMID: 36927607 Review.
Cited by
-
Identification of Terminal βGlcNAc on Brachyspira Species in Human Intestinal Spirochetosis.J Histochem Cytochem. 2024 Feb;72(2):71-78. doi: 10.1369/00221554231222963. Epub 2024 Jan 8. J Histochem Cytochem. 2024. PMID: 38189179 Free PMC article.
References
-
- Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nature Genet. 2006;38:787–93. - PubMed
-
- Samowitz WS, Albertsen H, Herrick J, Levin TR, Sweeney C, Murtaugh MA, et al. Evaluation of a large, population‐based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005;129:837–45. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

