Exploiting Real-Time Genomic Surveillance Data To Assess 4CMenB Meningococcal Vaccine Performance in Scotland, 2015 to 2022
- PMID: 37036356
- PMCID: PMC10127610
- DOI: 10.1128/mbio.00499-23
Exploiting Real-Time Genomic Surveillance Data To Assess 4CMenB Meningococcal Vaccine Performance in Scotland, 2015 to 2022
Abstract
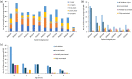
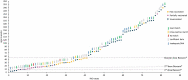
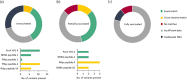
The United Kingdom implemented the first national infant immunization schedule for the meningococcal vaccine 4CMenB (Bexsero) in September 2015, targeting serogroup B invasive meningococcal disease (IMD). Bexsero contains four variable subcapsular proteins, and postimplementation IMD surveillance was necessary, as nonhomologous protein variants can evade Bexsero-elicited protection. We investigated postimplementation IMD cases reported in Scotland from 1 September 2015 to 30 June 2022. Patient demographics and vaccination status were combined with genotypic data from the causative meningococci, which were used to assess vaccine coverage with the meningococcal deduced vaccine antigen reactivity (MenDeVAR) index. Eighty-two serogroup B IMD cases occurred in children >5 years of age, 48 (58.5%) of which were in unvaccinated children and 34 (41%) of which were in children who had received ≥1 Bexsero dose. Fifteen of the 34 vaccinated children had received one dose, 17 had received two doses, and two had received three doses. For 39 cases, meningococcal sequence data were available, enabling MenDeVAR index deductions of vaccine-preventable (M-VP) and non-vaccine-preventable (M-NVP) meningococci. Notably, none of the 19 of the children immunized ≥2 times had IMD caused by M-VP meningococci, with 2 cases of NVP meningococci, and no deduction possible for 17. Among the 15 children partially vaccinated according to schedule (1 dose), 7 were infected by M-VP meningococci and 2 with M-NVP meningococci, with 6 for which deductions were not possible. Of the unvaccinated children with IMD, 40/48 were ineligible for vaccination and 20/48 had IMD caused by M-VP meningococci, with deductions not being possible for 14 meningococci. IMPORTANCE This study demonstrates the value of postimplementation genomic surveillance of vaccine-preventable pathogens in providing information on real-world vaccine performance. The data are consistent with 2 and 3 doses of Bexsero, delivered according to schedule, providing good protection against invasive disease caused by meningococci deduced from genomic data to be vaccine preventable. Single doses provide poorer protection to infants. In practical terms, these data can provide public health reassurance when vaccinated individuals develop IMD with non-vaccine-preventable variants. They further indicate that additional testing is needed on variants for which no immunological data exist to improve estimates of protection, although these data suggest that the uncharacterized variants are unlikely to be covered by Bexsero. Finally, the confirmation that incomplete or absent doses in infancy lead to reduced protection supports public health and general practitioners in promoting vaccination according to schedule.
Keywords: 4CMenB; BAST; Bexsero; MenDeVAR; WGS; breakthrough cases; genomics; meningococcal infections; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
'Be on the TEAM' Study (Teenagers Against Meningitis): protocol for a controlled clinical trial evaluating the impact of 4CMenB or MenB-fHbp vaccination on the pharyngeal carriage of meningococci in adolescents.BMJ Open. 2020 Oct 22;10(10):e037358. doi: 10.1136/bmjopen-2020-037358. BMJ Open. 2020. PMID: 33093030 Free PMC article.
-
Meningococcal Deduced Vaccine Antigen Reactivity (MenDeVAR) Index: a Rapid and Accessible Tool That Exploits Genomic Data in Public Health and Clinical Microbiology Applications.J Clin Microbiol. 2020 Dec 17;59(1):e02161-20. doi: 10.1128/JCM.02161-20. Print 2020 Dec 17. J Clin Microbiol. 2020. PMID: 33055180 Free PMC article.
-
Potential Coverage of the 4CMenB Vaccine against Invasive Serogroup B Neisseria meningitidis Isolated from 2009 to 2013 in the Republic of Ireland.mSphere. 2018 Aug 22;3(4):e00196-18. doi: 10.1128/mSphere.00196-18. mSphere. 2018. PMID: 30135218 Free PMC article.
-
Methods to evaluate serogroup B meningococcal vaccines: From predictions to real-world evidence.J Infect. 2020 Dec;81(6):862-872. doi: 10.1016/j.jinf.2020.07.034. Epub 2020 Jul 31. J Infect. 2020. PMID: 32745637 Review.
-
A comprehensive review of clinical and real-world safety data for the four-component serogroup B meningococcal vaccine (4CMenB).Expert Rev Vaccines. 2023 Jan-Dec;22(1):530-544. doi: 10.1080/14760584.2023.2222015. Expert Rev Vaccines. 2023. PMID: 37278390 Review.
Cited by
-
Immune interface interference vaccines: An evolution-informed approach to anti-bacterial vaccine design.Microb Biotechnol. 2024 Mar;17(3):e14446. doi: 10.1111/1751-7915.14446. Microb Biotechnol. 2024. PMID: 38536702 Free PMC article.
References
-
- Viner RM, Booy R, Johnson H, Edmunds WJ, Hudson L, Bedford H, Kaczmarski E, Rajput K, Ramsay M, Christie D. 2012. Outcomes of invasive meningococcal serogroup B disease in children and adolescents (MOSAIC): a case-control study. Lancet Neurol 11:774–783. doi:10.1016/S1474-4422(12)70180-1. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

