Schwann Cells Are Key Regulators of Corneal Epithelial Renewal
- PMID: 37036418
- PMCID: PMC10103722
- DOI: 10.1167/iovs.64.4.7
Schwann Cells Are Key Regulators of Corneal Epithelial Renewal
Abstract
Purpose: Corneal sensory nerves protect the cornea from injury. They are also thought to stimulate limbal stem cells (LSCs) to produce transparent epithelial cells constantly, enabling vision. In other organs, Schwann cells (SCs) associated with tissue-innervating axon terminals mediate tissue regeneration. This study defines the critical role of the corneal axon-ensheathing SCs in homeostatic and regenerative corneal epithelial cell renewal.
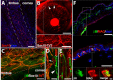
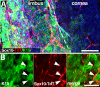
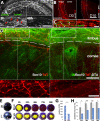
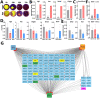
Methods: SC localization in the cornea was determined by in situ hybridization and immunohistochemistry with SC markers. In vivo SC visualization and/or ablation were performed in mice with inducible corneal SC-specific expression of tdTomato and/or Diphtheria toxin, respectively. The relative locations of SCs and LSCs were observed with immunohistochemical analysis of harvested genetically SC-prelabeled mouse corneas with LSC-specific antibodies. The correlation between cornea-innervating axons and the appearance of SCs was ascertained using corneal denervation in rats. To determine the limbal niche cellular composition and gene expression changes associated with innervation-dependent epithelial renewal, single-cell RNA sequencing (scRNA-seq) of dissociated healthy, de-epithelized, and denervated cornea limbi was performed.
Results: We observed limbal enrichment of corneal axon-associated myelinating and non-myelinating SCs. Induced local genetic ablation of SCs, although leaving corneal sensory innervation intact, markedly inhibited corneal epithelial renewal. scRNA-seq analysis (1) highlighted the transcriptional heterogenicity of cells populating the limbal niche, and (2) identified transcriptional changes associated with corneal innervation and during wound healing that model potential regulatory paracrine interactions between SCs and LSCs.
Conclusions: Limbal SCs are required for innervation-dependent corneal epithelial renewal.
Conflict of interest statement
Disclosure:
Figures

References
-
- Hanna C, Bicknell DS, O'Brien JE. Cell turnover in the adult human eye. Arch Ophthalmol. 1961; 65: 695–698. - PubMed
-
- Cenedella RJ, Fleschner CR.. Kinetics of corneal epithelium turnover in vivo. Studies of lovastatin. Invest Ophthalmol Vis Sci. 1990; 31: 1957–1962. - PubMed
-
- Schlotzer-Schrehardt U, Kruse FE.. Identification and characterization of limbal stem cells. Exp Eye Res. 2005; 81: 247–264. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

